Direct activation of a bacterial innate immune system by a viral capsid protein
- PMID: 36385533
- PMCID: PMC9712102
- DOI: 10.1038/s41586-022-05444-z
Direct activation of a bacterial innate immune system by a viral capsid protein
Abstract
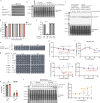
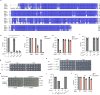
Bacteria have evolved diverse immunity mechanisms to protect themselves against the constant onslaught of bacteriophages1-3. Similar to how eukaryotic innate immune systems sense foreign invaders through pathogen-associated molecular patterns4 (PAMPs), many bacterial immune systems that respond to bacteriophage infection require phage-specific triggers to be activated. However, the identities of such triggers and the sensing mechanisms remain largely unknown. Here we identify and investigate the anti-phage function of CapRelSJ46, a fused toxin-antitoxin system that protects Escherichia coli against diverse phages. Using genetic, biochemical and structural analyses, we demonstrate that the C-terminal domain of CapRelSJ46 regulates the toxic N-terminal region, serving as both antitoxin and phage infection sensor. Following infection by certain phages, newly synthesized major capsid protein binds directly to the C-terminal domain of CapRelSJ46 to relieve autoinhibition, enabling the toxin domain to pyrophosphorylate tRNAs, which blocks translation to restrict viral infection. Collectively, our results reveal the molecular mechanism by which a bacterial immune system directly senses a conserved, essential component of phages, suggesting a PAMP-like sensing model for toxin-antitoxin-mediated innate immunity in bacteria. We provide evidence that CapRels and their phage-encoded triggers are engaged in a 'Red Queen conflict'5, revealing a new front in the intense coevolutionary battle between phages and bacteria. Given that capsid proteins of some eukaryotic viruses are known to stimulate innate immune signalling in mammalian hosts6-10, our results reveal a deeply conserved facet of immunity.
© 2022. The Author(s).
Conflict of interest statement
A.G.-P. is co-founder and stockholder of Santero Therapeutics. the other authors declare no competing interests.
Figures









Comment in
-
Phage capsid recognition triggers activation of a bacterial toxin-antitoxin defense system.Mol Cell. 2023 Jan 19;83(2):165-166. doi: 10.1016/j.molcel.2022.12.020. Mol Cell. 2023. PMID: 36669478
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

