Phase 1 safety trial of a natural product cocktail with antibacterial activity in human volunteers
- PMID: 36385621
- PMCID: PMC9667429
- DOI: 10.1038/s41598-022-22700-4
Phase 1 safety trial of a natural product cocktail with antibacterial activity in human volunteers
Abstract
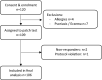
New antibiotics are urgently needed to reduce the health burden of antibiotic-resistant bacterial infection. Natural products (NPs) derived from plants and animals are a current focus of research seeking to discover new antibacterial molecules with clinical potential. A cocktail of NPs based on a medieval remedy for eye infection eliminated biofilms of several highly antibiotic-resistant bacterial species in laboratory studies, and had a promising safety profile in vitro and in a mouse model. A necessary prelude to refining this remedy into a defined, synthetic mixture suitable for testing with wound infections is to firstly establish safety when applied to healthy human skin. We aimed to assess skin-related outcomes of the preparation in a sample of healthy volunteers. This prospective, single arm, non-randomised Phase I clinical trial consisted of a single patch test intervention with 48-h follow-up. Volunteers were staff, students and members of the public recruited from the University of Warwick and surrounding locality. Adults aged 18-79 years, with no history of severe immunity-related disease, diabetes, recent infection, or known pregnancy were eligible. A 100 µl application of a filter-sterilised NP mixture, comprising ground garlic, onion, white wine and bovine bile, was applied to skin on the upper arm and covered with a dressing. The primary outcome was skin-related adverse events over 48 h. Digital photographs were captured where bothersome, salve-related events were reported. 109 volunteers, aged 18-77 years, were recruited between June and July 2021. Sample mean age was 37.6 (SD 16.1) years, and 63 (58%) participants were female. Outcome data were obtained for 106/109 (97%); two participants were lost to follow-up and one removed the skin patch after nine hours due to a bothersome garlic odour. Twenty-one (19.8%) participants reported any patch-test related sign or symptom; of these 14 (13.2%) participants reported minor events related to the salve, including itchiness, redness, or garlic odour. No serious events were reported. We found no evidence of serious skin-related adverse events related to the NP preparation.Trial registration: International Standard Randomised Controlled Trial Number (ISRCTN10773579). Date registered: 08/01/2021.
© 2022. Crown.
Conflict of interest statement
The authors declare no competing interests.
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib to prevent respiratory insufficiency progression in onco-hematological patients affected with COVID19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Feb 5;22(1):116. doi: 10.1186/s13063-021-05072-4. Trials. 2021. PMID: 33546739 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Interventions to reduce Staphylococcus aureus in the management of eczema.Cochrane Database Syst Rev. 2019 Oct 29;2019(10):CD003871. doi: 10.1002/14651858.CD003871.pub3. Cochrane Database Syst Rev. 2019. PMID: 31684694 Free PMC article.
-
Interventions for bacterial folliculitis and boils (furuncles and carbuncles).Cochrane Database Syst Rev. 2021 Feb 26;2(2):CD013099. doi: 10.1002/14651858.CD013099.pub2. Cochrane Database Syst Rev. 2021. PMID: 33634465 Free PMC article.
Cited by
-
Reviving the past for a healthier future: ancient molecules and remedies as a solution to the antibiotic crisis.Future Microbiol. 2025 Apr;20(5):429-441. doi: 10.1080/17460913.2025.2476290. Epub 2025 Mar 18. Future Microbiol. 2025. PMID: 40099865 Free PMC article. Review.
-
A case study of the Ancientbiotics collaboration.Patterns (N Y). 2022 Dec 9;3(12):100632. doi: 10.1016/j.patter.2022.100632. eCollection 2022 Dec 9. Patterns (N Y). 2022. PMID: 36569547 Free PMC article. Review.
-
Prokaryotic Expression and Functional Verification of Antimicrobial Peptide LRGG.Int J Mol Sci. 2024 Jun 27;25(13):7072. doi: 10.3390/ijms25137072. Int J Mol Sci. 2024. PMID: 39000180 Free PMC article.
References
-
- World Health Organization. Global action plan on antimicrobial resistance. IBSN: 978 92 4 150976 3. 2015. - PubMed
-
- The Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Available at amr-review.org. 2014.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous