Association between SARS-CoV-2 RNAemia and dysregulated immune response in acutely ill hospitalized COVID-19 patients
- PMID: 36385627
- PMCID: PMC9667450
- DOI: 10.1038/s41598-022-23923-1
Association between SARS-CoV-2 RNAemia and dysregulated immune response in acutely ill hospitalized COVID-19 patients
Abstract
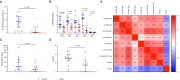
Severe/critical COVID-19 is associated with immune dysregulation and plasmatic SARS-CoV-2 detection (i.e. RNAemia). We detailed the association of SARS-CoV-2 RNAemia with immune responses in COVID-19 patients at the end of the first week of disease. We enrolled patients hospitalized in acute phase of ascertained SARS-CoV-2 pneumonia, and evaluated SARS-CoV-2 RNAemia, plasmatic cytokines, activated/pro-cytolytic T-cells phenotypes, SARS-CoV-2-specific cytokine-producing T-cells (IL-2, IFN-γ, TNF-α, IL-4, IL-17A), simultaneous Th1-cytokines production (polyfunctionality) and amount (iMFI). The humoral responses were assessed with anti-S1/S2 IgG, anti-RBD total-Ig, IgM, IgA, IgG1 and IgG3, neutralization and antibody-dependent cellular cytotoxicity (ADCC). Out of 54 patients, 27 had detectable viremia (viremic). Albeit comparable age and co-morbidities, viremic more frequently required ventilatory support, with a trend to higher death. Viremic displayed higher pro-inflammatory cytokines (IFN-α, IL-6), lower activated T-cells (HLA-DR+CD38+), lower functional SARS-CoV-2-specific T-cells (IFN-γ+CD4+, TNF-α+CD8+, IL-4+CD8+, IL-2+TNF-α+CD4+, and IL-2+TNF-α+CD4+ iMFI) and SARS-CoV-2-specific Abs (anti-S IgG, anti-RBD total-Ig, IgM, IgG1, IgG3; ID50, %ADCC). These data suggest a link between SARS-CoV-2 RNAemia at the end of the first stage of disease and immune dysregulation. Whether high ab initium viral burden and/or intrinsic host factors contribute to immune dysregulation in severe COVID-19 remains to be elucidated, to further inform strategies of targeted therapeutic interventions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

