The transforming growth factor beta ligand TIG-2 modulates the function of neuromuscular junction and muscle energy metabolism in Caenorhabditis elegans
- PMID: 36385772
- PMCID: PMC9650414
- DOI: 10.3389/fnmol.2022.962974
The transforming growth factor beta ligand TIG-2 modulates the function of neuromuscular junction and muscle energy metabolism in Caenorhabditis elegans
Abstract
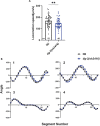
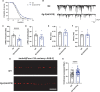
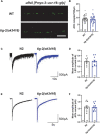
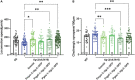
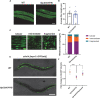
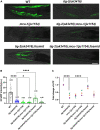
Deciphering the physiological function of TGF-β (the transforming growth factor beta) family ligands is import for understanding the role of TGF-β in animals' development and aging. Here, we investigate the function of TIG-2, one of the ligands in Caenorhabditis elegans TGF-β family, in animals' behavioral modulation. Our results show that a loss-of-function mutation in tig-2 gene result in slower locomotion speed in the early adulthood and an increased density of cholinergic synapses, but a decreased neurotransmitter release at neuromuscular junctions (NMJs). Further tissue-specific rescue results reveal that neuronal and intestinal TIG-2 are essential for the formation of cholinergic synapses at NMJs. Interestingly, tig-2(ok3416) mutant is characterized with reduced muscle mitochondria content and adenosine triphosphate (ATP) production, although the function of muscle acetylcholine receptors and the morphology muscle fibers in the mutant are comparable to that in wild-type animals. Our result suggests that TIG-2 from different neuron and intestine regulates worm locomotion by modulating synaptogenesis and neurotransmission at NMJs, as well as energy metabolism in postsynaptic muscle cells.
Keywords: Caenorhabditis elegans; TIG-2; acetylcholine; locomotion; mitochondria; neuromuscular junction.
Copyright © 2022 Cheng, Yan, Su and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
TGF-β ligand cross-subfamily interactions in the response of Caenorhabditis elegans to a bacterial pathogen.PLoS Genet. 2024 Jun 14;20(6):e1011324. doi: 10.1371/journal.pgen.1011324. eCollection 2024 Jun. PLoS Genet. 2024. PMID: 38875298 Free PMC article.
-
Sexually Dimorphic Neurotransmitter Release at the Neuromuscular Junction in Adult Caenorhabditis elegans.Front Mol Neurosci. 2022 Jan 31;14:780396. doi: 10.3389/fnmol.2021.780396. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35173578 Free PMC article.
-
TGF-β Ligand Cross-Subfamily Interactions in the Response of Caenorhabditis elegans to Bacterial Pathogens.bioRxiv [Preprint]. 2023 Aug 9:2023.05.05.539606. doi: 10.1101/2023.05.05.539606. bioRxiv. 2023. Update in: PLoS Genet. 2024 Jun 14;20(6):e1011324. doi: 10.1371/journal.pgen.1011324. PMID: 37215035 Free PMC article. Updated. Preprint.
-
Practical Anatomy of the Neuromuscular Junction in Health and Disease.Neurol Clin. 2018 May;36(2):231-240. doi: 10.1016/j.ncl.2018.01.009. Neurol Clin. 2018. PMID: 29655446 Free PMC article. Review.
-
Glutamate at the Vertebrate Neuromuscular Junction: From Modulation to Neurotransmission.Cells. 2019 Aug 28;8(9):996. doi: 10.3390/cells8090996. Cells. 2019. PMID: 31466388 Free PMC article. Review.
Cited by
-
TGF-β pathways in aging and immunity: lessons from Caenorhabditis elegans.Front Genet. 2023 Sep 5;14:1220068. doi: 10.3389/fgene.2023.1220068. eCollection 2023. Front Genet. 2023. PMID: 37732316 Free PMC article. Review.
-
PCDH7 as the key gene related to the co-occurrence of sarcopenia and osteoporosis.Front Genet. 2023 Jul 5;14:1163162. doi: 10.3389/fgene.2023.1163162. eCollection 2023. Front Genet. 2023. PMID: 37476411 Free PMC article.
References
LinkOut - more resources
Full Text Sources

