Preparation and Characterization of Aprepitant Solid Dispersion with HPMCAS-LF
- PMID: 36385804
- PMCID: PMC9647728
- DOI: 10.1021/acsomega.2c04021
Preparation and Characterization of Aprepitant Solid Dispersion with HPMCAS-LF
Abstract
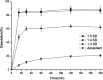
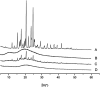
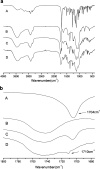
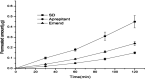
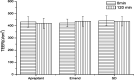
This study focused on improving the physicochemical characteristics of aprepitant with poor water solubility by preparing solid dispersion (SD). To prepare the SD with HPMCAS-LF, the solvent evaporation method was applied. Based on dissolution analysis, the dissolution rate of SD increased by five times compared with aprepitant. In addition, scanning electron microscopy (SEM), X-ray powder diffraction (XRPD), and differential scanning calorimetry (DSC) results suggested the presence of amorphous-form aprepitant inside SD. According to Fourier transform infrared (FTIR) spectroscopy, intermolecular hydrogen bonds were detected between polymer and aprepitant. The Caco-2 cell experiment proved that SD did not lower the transepithelial electrical resistance (TEER) values but improved the permeation amount of aprepitant. Additionally, the SD of aprepitant displayed excellent stability.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Sorbera L. A.; Castañer J.; Bayés M.; Silvestre J. Aprepitant and L-758298. Drugs Future 2002, 27, 211–222. 10.1358/dof.2002.027.03.661047. - DOI
-
- Nama S.; Chandu B. R.; Awen B. Z.; Khagga M. Development and Validation of a New RP-HPLC Method for the Determination of Aprepitant in Solid Dosage Forms. Trop. J. Pharm. Res. 2011, 10, 491–497. 10.4314/tjpr.v10i4.15. - DOI
-
- Cocquyt V.; Van Belle S.; Belle S. V.; Reinhardt R. R.; Decramer M.; O’Brien M.; O’Brien M.; Schellens J.; Schelle J. H.; Borms M.; Verbeke L.; Verbeke L.; Van A. F.; Van Aelst F.; De S. M.; De Smet M.; Carides A. D.; Carides A.; Eldridge K.; Eldridge K.; Gretz B. J. Comparison of L-758,298, a prodrug for the selective neurokinin-1 antagonist, L-754,030, with ondansetron for the prevention of cisplatin-induced emesis. Eur. J. Cancer. 2001, 37, 835–842. 10.1016/S0959-8049(00)00416-0. - DOI - PubMed
-
- Wu Y.; Loper A.; Landis E.; Hettrick L.; Novak L.; Lynn K.; Chen C.; Thompson K.; Higgins R.; Batra U.; Shelukar S.; Kwei G.; Storey D. The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: a Beagle dog model predicts improved bioavailability and diminished food effect on absorption in human. Int. J. Pharm. 2004, 285, 135–146. 10.1016/j.ijpharm.2004.08.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources

