Review on CO2 Capture Using Amine-Functionalized Materials
- PMID: 36385890
- PMCID: PMC9647976
- DOI: 10.1021/acsomega.2c03385
Review on CO2 Capture Using Amine-Functionalized Materials
Abstract
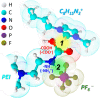
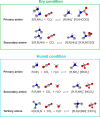
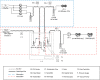
CO2 capture from industry sectors or directly from the atmosphere is drawing much attention on a global scale because of the drastic changes in the climate and ecosystem which pose a potential threat to human health and life on Earth. In the past decades, CO2 capture technology relied on classical liquid amine scrubbing. Due to its high energy consumption and corrosive property, CO2 capture using solid materials has recently come under the spotlight. A variety of porous solid materials were reported such as zeolites and metal-organic frameworks. However, amine-functionalized porous materials outperform all others in terms of CO2 adsorption capacity and regeneration efficiency. This review provides a brief overview of CO2 capture by various amines and mechanistic aspects for newcomers entering into this field. This review also covers a state-of-the-art regeneration method, visible/UV light-triggered CO2 desorption at room temperature. In the last section, the current issues and future perspectives are summarized.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Design of Amine-Containing Nanoporous Materials for Postcombustion CO2 Capture from Engineering Perspectives.Acc Chem Res. 2023 Nov 7;56(21):2887-2897. doi: 10.1021/acs.accounts.3c00326. Epub 2023 Oct 12. Acc Chem Res. 2023. PMID: 37824727
-
Carbon Capture Using Porous Silica Materials.Nanomaterials (Basel). 2023 Jul 11;13(14):2050. doi: 10.3390/nano13142050. Nanomaterials (Basel). 2023. PMID: 37513061 Free PMC article. Review.
-
Adsorption of CO2 on amine-functionalized green metal-organic framework: an interaction between amine and CO2 molecules.Environ Sci Pollut Res Int. 2019 Dec;26(36):36214-36225. doi: 10.1007/s11356-019-06717-3. Epub 2019 Nov 11. Environ Sci Pollut Res Int. 2019. PMID: 31713140
-
An overview on trace CO2 removal by advanced physisorbent materials.J Environ Manage. 2020 Feb 1;255:109874. doi: 10.1016/j.jenvman.2019.109874. Epub 2019 Nov 26. J Environ Manage. 2020. PMID: 31783210 Review.
-
Stability of amine-functionalized CO2 adsorbents: a multifaceted puzzle.Chem Soc Rev. 2019 Jun 17;48(12):3320-3405. doi: 10.1039/c8cs00877a. Chem Soc Rev. 2019. PMID: 31149678 Review.
Cited by
-
Equivariant Neural Networks Utilizing Molecular Clusters for Accurate Molecular Crystal Lattice Energy Predictions.ACS Omega. 2024 Sep 11;9(38):40269-40282. doi: 10.1021/acsomega.4c07434. eCollection 2024 Sep 24. ACS Omega. 2024. PMID: 39346862 Free PMC article.
-
Assessment of Long-Term Degradation of Adsorbents for Direct Air Capture by Ozonolysis.J Phys Chem C Nanomater Interfaces. 2024 Dec 20;129(1):899-909. doi: 10.1021/acs.jpcc.4c07054. eCollection 2025 Jan 9. J Phys Chem C Nanomater Interfaces. 2024. PMID: 39811441 Free PMC article.
-
Isothermal Dehydrogenation of Ammonia Borane: Insights into BNH Polymers and Challenges in Regeneration.Chem Asian J. 2025 May 2;20(9):e202500140. doi: 10.1002/asia.202500140. Epub 2025 Mar 12. Chem Asian J. 2025. PMID: 40019285 Free PMC article.
-
Utilization of waste tire derived activated carbon as CO2 capture and photocatalyst for CO2 conversion.Sci Rep. 2024 Jul 24;14(1):17100. doi: 10.1038/s41598-024-67631-4. Sci Rep. 2024. PMID: 39048643 Free PMC article.
-
Organic and Metal-Organic Polymer-Based Catalysts-Enfant Terrible Companions or Good Assistants?Molecules. 2024 Sep 29;29(19):4623. doi: 10.3390/molecules29194623. Molecules. 2024. PMID: 39407552 Free PMC article. Review.
References
-
- Zhao X. Y.; Qian J. L.; Wang J.; He Q. Y.; Wang Z. L.; Chen C. Z. Using a tree ring delta C-13 annual series to reconstruct atmospheric CO2 concentration over the past 300 years. Pedosphere 2006, 16 (3), 371–379. 10.1016/S1002-0160(06)60065-9. - DOI
-
- Kawahata H.; Fujita K.; Iguchi A.; Inoue M.; Iwasaki S.; Kuroyanagi A.; Maeda A.; Manaka T.; Moriya K.; Takagi H.; Toyofuku T.; Yoshimura T.; Suzuki A. Perspective on the response of marine calcifiers to global warming and ocean acidification-Behavior of corals and foraminifera in a high CO2 world ″hot house″. Prog. Earth Planet. Sci. 2019, 10.1186/s40645-018-0239-9. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

