Advancing New Chemical Modalities into Clinical Studies
- PMID: 36385931
- PMCID: PMC9661701
- DOI: 10.1021/acsmedchemlett.2c00375
Advancing New Chemical Modalities into Clinical Studies
Abstract
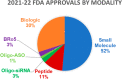
Drug discovery and development has experienced an incredible paradigm shift in the past two decades. What once was considered a predominant R&D landscape of small molecules within a prescribed properties and mechanism space now includes an innovative wave of new chemical modalities. Scientists in the pharmaceutical industry can now strategize across a variety of modalities to find the best option to modulate a given target and provide treatment for a specific disease. We have witnessed a remarkable change not only in molecular design but also in creative approaches to drug delivery that have enabled advancement of novel modalities to clinical studies. In this Microperspective, we evaluate the critical differences between traditional small molecules and beyond rule of 5 compounds, peptides, oligonucleotides, and biologics for advancing into development, particularly their pharmacokinetic profiles and drug delivery strategies.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.N.N. discloses that his research is funded by AbbVie. He is a consultant for Axonis and on the SAB of Sionna Therapeutics.
Figures



References
-
- Goodsell D. S.; Zardecki C.; Di Costanzo L.; Duarte J. M.; Hudson B. P.; Persikova I.; Segura J.; Shao C.; Voigt M.; Westbrook J. D.; Young J. Y.; Burley S. K. RCSB Protein Data Bank: Enabling biomedical research and drug discovery. Protein Sci. 2020, 29, 52–65. 10.1002/pro.3730. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

