Poly(aniline- co-melamine)@MnFe2O4 nanocatalyst for the synthesis of 4,4'-(arylmethylene) bis (1H-pyrazole-5-ol) derivatives, and 1,4- dihydropyrano[2,3- c]pyrazoles and evaluation of their antioxidant, and anticancer activities
- PMID: 36385997
- PMCID: PMC9649443
- DOI: 10.3389/fchem.2022.1046120
Poly(aniline- co-melamine)@MnFe2O4 nanocatalyst for the synthesis of 4,4'-(arylmethylene) bis (1H-pyrazole-5-ol) derivatives, and 1,4- dihydropyrano[2,3- c]pyrazoles and evaluation of their antioxidant, and anticancer activities
Abstract
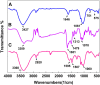
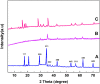
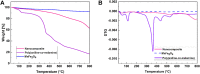
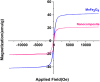
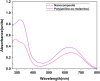
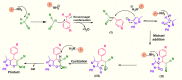
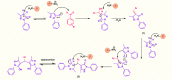
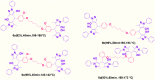
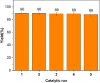
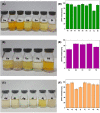
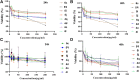
In this work, magnetic poly(aniline-co-melamine) nanocomposite as an efficient heterogeneous polymer-based nanocatalyst was fabricated in two steps. First, poly(aniline-co-melamine) was synthesized through the chemical oxidation by ammonium persulfate, then the magnetic nanocatalyst was successfully prepared from the in-situ coprecipitation method in the presence of poly(aniline-co-melamine). The resulting poly(aniline-co-melamine)@MnFe2O4 was characterized by FTIR, FESEM, XRD, VSM, EDX, TGA, and UV-vis analyses. The catalytic activity of poly(aniline-co-melamine)@MnFe2O4 was investigated in the synthesis of 4,4'-(arylmethylene)bis(1H-pyrazole-5-ol) derivatives, and new alkylene bridging bis 4,4'-(arylmethylene)bis(1H-pyrazole-5-ol) derivatives in excellent yields. The yield of 1,4-dihydropyrano[2,3-c]pyrazoles, 4,4'-(arylmethylene)bis(1H-pyrazol-5-ol), yields, and new alkylene bridging bis 4,4'-(arylmethylene)bis(1H-pyrazol-5-ol) derivatives were obtained 89%-96%, 90%-96%, and 92%-96%, respectively. The poly(aniline-co-melamine)@MnFe2O4 nanocatalyst can be recycled without pre-activation and reloaded up to five consecutive runs without a significant decrease in its efficiency. In addition, the antioxidant activity of some derivatives was evaluated by DPPH assay. Results showed that the maximum antioxidant activity of 4,4'-(arylmethylene)bis(1H-pyrazole-5-ol) derivatives and 1,4-dihydropyrano[2,3-c]pyrazoles were 75% and 90%, respectively. Furthermore, 4,4'-(arylmethylene)bis(1H-pyrazole-5-ol) derivatives and 1,4-dihydropyrano[2,3-c]pyrazoles showed good potential for destroying colon cancer cell lines. Consequently, the poly(aniline-co-melamine)@MnFe2O4 nanocomposite is an excellent catalyst for green chemical processes owing to its high catalytic activity, stability, and reusability.
Keywords: anticancer; antioxidant; nanocatalyst; poly(aniline-co-melamine)@MnFe2O4 nanocomposite; pyrazole derivatives.
Copyright © 2022 Mirani Nezhad, Pourmousavi, Nazarzadeh Zare, Heidari, Manoochehri and Sharifi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer AM declared a past co-authorship with the author EN to the handling editor.
Figures

Similar articles
-
Superparamagnetic Poly(aniline-co-m-phenylenediamine)@Fe3O4 Nanocomposite as an Efficient Heterogeneous Catalyst for the Synthesis of 1H-pyrazolo[1,2-a]pyridazine-5,8-diones & 1H-pyrazolo[1,2-b]phthalazine-5, 10-diones Derivatives.Curr Org Synth. 2022 Mar 3;19(2):246-266. doi: 10.2174/1570179418666211104143736. Curr Org Synth. 2022. PMID: 34736384
-
Non-Covalent Supported of l-Proline on Graphene Oxide/Fe₃O₄ Nanocomposite: A Novel, Highly Efficient and Superparamagnetically Separable Catalyst for the Synthesis of Bis-Pyrazole Derivatives.Molecules. 2018 Feb 5;23(2):330. doi: 10.3390/molecules23020330. Molecules. 2018. PMID: 29401720 Free PMC article.
-
Magnetic poly(1,8-diaminonaphthalene)-nickel nanocatalyst for the synthesis of antioxidant and antibacterial isoxazole-5(4H)-ones derivatives.Heliyon. 2023 May 4;9(5):e15886. doi: 10.1016/j.heliyon.2023.e15886. eCollection 2023 May. Heliyon. 2023. PMID: 37206030 Free PMC article.
-
Pyrazole Containing Anti-HIV Agents: An Update.Med Chem. 2022;18(8):831-846. doi: 10.2174/1573406418666220106163846. Med Chem. 2022. PMID: 34994333 Review.
-
Recent Developments in Reactions and Catalysis of Protic Pyrazole Complexes.Molecules. 2023 Apr 17;28(8):3529. doi: 10.3390/molecules28083529. Molecules. 2023. PMID: 37110763 Free PMC article. Review.
Cited by
-
Novel modified magnetic graphene oxide as an efficient and green catalyst for one-pot synthesis of pyrazoles.Sci Rep. 2025 Apr 24;15(1):14263. doi: 10.1038/s41598-025-97884-6. Sci Rep. 2025. PMID: 40274851 Free PMC article.
-
Silver-assisted reduction of nitroarenes by an Ag-embedded curcumin/melamine-functionalized magnetic nanocatalyst.Sci Rep. 2023 Mar 30;13(1):5225. doi: 10.1038/s41598-023-32560-1. Sci Rep. 2023. PMID: 36997564 Free PMC article.
References
-
- Al-Hasani T. J., Mihsen H. H., Hello K. M., Adam F. (2017). Catalytic esterification via silica immobilized p-phenylenediamine and dithiooxamide solid catalysts. Arab. J. Chem. 10, S1492–S1500. 10.1016/j.arabjc.2013.04.030 - DOI
-
- Alizadeh E., Baseri H. (2022). Photocatalytic degradation of sumatriptan succinate by ZnO, Fe doped ZnO and TiO2-ZnO nanocatalysts. Mat. Chem. Horizons 1, 7–21. 10.22128/mch.2022.534.1002 - DOI
-
- Bououdina M., Manoharan C. (2020). Dependence of structure/morphology on electrical/magnetic properties of hydrothermally synthesised cobalt ferrite nanoparticles. J. Magn. Magn. Mat. 493, 165703. 10.1016/j.jmmm.2019.165703 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

