Chemodivergent photocatalytic access to 1-pyrrolines and 1-tetralones involving switchable C(sp3)-H functionalization
- PMID: 36385998
- PMCID: PMC9641198
- DOI: 10.3389/fchem.2022.1058596
Chemodivergent photocatalytic access to 1-pyrrolines and 1-tetralones involving switchable C(sp3)-H functionalization
Abstract
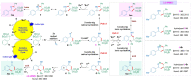
A chemodivergent photocatalytic approach to 1-pyrrolines and 1-tetralones from alkyl bromides and vinyl azides has been developed through chemoselectively controllable intermolecular [3 + 2] and [4 + 2] cyclization. This photoredox-neutral two-component protocol involves intermolecular radical addition and switchable distal C(sp3)-H functionalization enabled by iminyl radical-mediated 1,5-hydrogen atom transfer. Meanwhile, chemoselectivity between C(sp3)-N bond formation and C(sp3)-C(sp2) bond formation is precisely switched by photocatalysts (Ru(bpy)3(PF6)2 vs. fac-Ir(ppy)3) and additives (base vs. acid).
Keywords: 1-pyrrolines; 1-tetralones; [3+2] cyclization; [4+2] cyclization; hydrogen atom transfer.
Copyright © 2022 Tu, Qi, Li, Zhang, Zhang, Wei, Yang, Wei, Du and Yi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Direct C(sp3)-N Radical Coupling: Photocatalytic C-H Functionalization by Unconventional Intermolecular Hydrogen Atom Transfer to Aryl Radical.Org Lett. 2020 Aug 7;22(15):6112-6116. doi: 10.1021/acs.orglett.0c02179. Epub 2020 Jul 20. Org Lett. 2020. PMID: 32687373
-
Visible-light photocatalytic radical alkenylation of α-carbonyl alkyl bromides and benzyl bromides.Chemistry. 2013 Apr 15;19(16):5120-6. doi: 10.1002/chem.201203694. Epub 2013 Feb 20. Chemistry. 2013. PMID: 23426910
-
α-Aminoxy-Acid-Auxiliary-Enabled Intermolecular Radical γ-C(sp3 )-H Functionalization of Ketones.Angew Chem Int Ed Engl. 2018 Feb 5;57(6):1692-1696. doi: 10.1002/anie.201712066. Epub 2018 Jan 15. Angew Chem Int Ed Engl. 2018. PMID: 29266582
-
Direct C(sp3)-H functionalization with aryl and alkyl radicals as intermolecular hydrogen atom transfer (HAT) agents.Chem Commun (Camb). 2024 Oct 8;60(81):11450-11465. doi: 10.1039/d4cc03383c. Chem Commun (Camb). 2024. PMID: 39268687 Review.
-
Radical carbon-carbon bond formations enabled by visible light active photocatalysts.Chimia (Aarau). 2012;66(6):394-8. doi: 10.2533/chimia.2012.394. Chimia (Aarau). 2012. PMID: 22871282 Review.
References
-
- Chen C., Zhao J., Shi X., Liu L., Zhu Y.-P., Sun W., et al. (2020). Recent advances in cyclization reactions of unsaturated oxime esters (ethers): Synthesis of versatile functionalized nitrogen-containing scaffolds. Org. Chem. Front. 7, 1948–1969. 10.1039/d0qo00397b - DOI
-
- Dong D.-Q., Song J.-C., Yang S.-H., Qin Q.-X., Wang Z.-L., Zhang E.-X., et al. (2022). C(sp3)−H bond functionalization of oximes derivatives via 1, 5−hydrogen atom transfer induced by iminyl radical. Chin. Chem. Lett. 33, 1199–1206. 10.1016/j.cclet.2021.08.067 - DOI
LinkOut - more resources
Full Text Sources

