Cannabidiol for neurodegenerative disorders: A comprehensive review
- PMID: 36386183
- PMCID: PMC9640911
- DOI: 10.3389/fphar.2022.989717
Cannabidiol for neurodegenerative disorders: A comprehensive review
Abstract
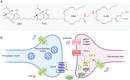
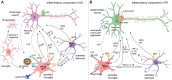
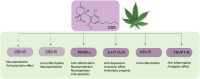
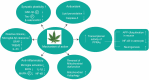
Despite the significant advances in neurology, the cure for neurodegenerative conditions remains a formidable task to date. Among various factors arising from the complex etiology of neurodegenerative diseases, neuroinflammation and oxidative stress play a major role in pathogenesis. To this end, some phytocannabinoids isolated from Cannabis sativa (widely known as marijuana) have attracted significant attention as potential neurotherapeutics. The profound effect of ∆9-tetrahydrocannabinol (THC), the major psychoactive component of cannabis, has led to the discovery of the endocannabinoid system as a molecular target in the central nervous system (CNS). Cannabidiol (CBD), the major non-psychoactive component of cannabis, has recently emerged as a potential prototype for neuroprotective drug development due to its antioxidant and anti-inflammatory properties and its well-tolerated pharmacological behavior. This review briefly discusses the role of inflammation and oxidative stress in neurodegeneration and demonstrates the neuroprotective effect of cannabidiol, highlighting its general mechanism of action and disease-specific pathways in Parkinson's disease (PD) and Alzheimer's disease (AD). Furthermore, we have summarized the preclinical and clinical findings on the therapeutic promise of CBD in PD and AD, shed light on the importance of determining its therapeutic window, and provide insights into identifying promising new research directions.
Keywords: Alzheimer’s disease; Parkinson’s disease; cannabidiol; neurodegenerative diseases; neuroinflammation; oxidative stress.
Copyright © 2022 Bhunia, Kolishetti, Arias, Vashist and Nair.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aso E., Andrés-Benito P., Carmona M., Maldonado R., Ferrer I. (2016). Cannabinoid receptor 2 participates in amyloid-β processing in a mouse model of alzheimer's disease but plays a minor role in the therapeutic properties of a cannabis-based medicine. J. Alzheimers Dis. 51 (2), 489–500. 10.3233/JAD-150913 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

