Potential of nanoformulations in malaria treatment
- PMID: 36386185
- PMCID: PMC9645116
- DOI: 10.3389/fphar.2022.999300
Potential of nanoformulations in malaria treatment
Abstract
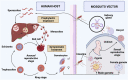
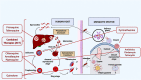
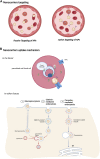
Malaria is caused by the protozoan Plasmodium sp and affects millions of people worldwide. Its clinical form ranges from asymptomatic to potentially fatal and severe. Current treatments include single drugs such as chloroquine, lumefantrine, primaquine, or in combination with artemisinin or its derivatives. Resistance to antimalarial drugs has increased; therefore, there is an urgent need to diversify therapeutic approaches. The disease cycle is influenced by biological, social, and anthropological factors. This longevity and complexity contributes to the records of drug resistance, where further studies and proposals for new therapeutic formulations are needed for successful treatment of malaria. Nanotechnology is promising for drug development. Preclinical formulations with antimalarial agents have shown positive results, but only a few have progressed to clinical phase. Therefore, studies focusing on the development and evaluation of antimalarial formulations should be encouraged because of their enormous therapeutic potential.
Keywords: infectious disease; malaria; nanotechnology; pre-clinical study; treatment.
Copyright © 2022 Chaves, Portugal Tavares de Moraes, Regina Ferrarini, Noé da Fonseca, Silva and Gonçalves-de-Albuquerque.
Conflict of interest statement
Author FN was employed by Empresa Brasileira de Pesquisa Agropecuária, EMBRAPA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Agbo C. P., Ugwuanyi T. C., Ugwuoke W. I., Mcconville C., Attama A. A., Ofokansi K. C. (2021). Intranasal artesunate-loaded nanostructured lipid carriers: A convenient alternative to parenteral formulations for the treatment of severe and cerebral malaria. J. Control. Release 334, 224–236. 10.1016/j.jconrel.2021.04.020 - DOI - PubMed
-
- Akpa P. A., Ugwuoke J. A., Attama A. A., Nnenna Ugwu C., Nneoma Ezeibe E., Momoh M. A., et al. (2020). Improved antimalarial activity of caprol-based nanostructured lipid carriers encapsulating artemether-lumefantrine for oral administration. Afr. Health Sci. 20 (4), 1679–1697. 10.4314/ahs.v20i4.20 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

