COVID-19 Vaccination reduced pneumonia severity
- PMID: 36386765
- PMCID: PMC9650570
- DOI: 10.1016/j.ejro.2022.100456
COVID-19 Vaccination reduced pneumonia severity
Abstract
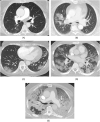
Purpose: To investigate the effect of vaccinations and boosters on the severity of COVID-19 pneumonia on CT scans during the period of Delta and Omicron variants.
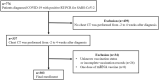
Methods: Retrospectively studied were 303 patients diagnosed with COVID-19 between July 2021 and February 2022, who had obtained at least one CT scan within 6 weeks around the COVID-19 diagnosis (-2 to +4 weeks). The severity of pneumonia was evaluated with a 6-point scale Pneumonia Score. The association between demographic and clinical data and vaccination status (booster/additional vaccination, complete vaccination and un-vaccination) and the difference between Pneumonia Scores by vaccination status were investigated.
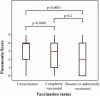
Results: Of 303 patients (59.4 ± 16.3 years; 178 females), 62 (20 %) were in the booster/additional vaccination group, 117 (39 %) in the complete vaccination group, and 124 (41 %) in the unvaccinated group. Interobserver agreement of the Pneumonia Score was high (weighted kappa score = 0.875). Patients in the booster/additionally vaccinated group tended to be older (P = 0.0085) and have more underlying comorbidities (P < 0.0001), and the Pneumonia Scores were lower in the booster/additionally vaccinated [median 2 (IQR 0-4)] and completely vaccinated groups [median 3 (IQR 1-4)] than those in the unvaccinated group [median 4 (IQR 2-4)], respectively (P < 0.0001 and P < 0.0001, respectively). A multivariable linear analysis adjusted for confounding factors confirmed the difference.
Conclusion: Vaccinated patients, with or without booster/additional vaccination, had milder COVID-19 pneumonia on CT scans than unvaccinated patients during the period of Delta and Omicron variants. This study supports the efficacy of the vaccine against COVID-19 from a radiological perspective.
Keywords: COVID-19; COVID-19, coronavirus disease 2019; CT; DAD, diffuse alveolar damage; GGO, ground-glass opacity; Lung; OP, organizing pneumonia; Pneumonia; RT-PCR, reverse transcription-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Vaccination; mRNA, messenger ribonucleic acid.
© 2022 The Authors.
Conflict of interest statement
M.N. reports research grant to the institution from Merck, Canon Medical Systems, AstraZeneca, and Daiichi Sankyo; and consulting fees from Daiichi Sankyo and AstraZeneca, outside the submitted work. C.R.G.G. reports stock ownership of GSK, Roche, and Novartis, outside the submitted work. G.M.H. reports consulting fees from Boehringer Ingelheim, Gerson Lehrman Group, and Chugai Pharmaceuticals, outside the submitted work. B.D.L reports grants or contacts from NIH, Pieris Pharmaceuticals, SRA, and Sanofi; royalties or licenses from Propeller Health; consulting fees from AstraZeneca, NControl, Cartesian, Novartis, Gossamer Bio, and Thetis Pharmaceuticals; participation on a data safety monitoring board or advisory board from NIAID; and stock or stock options from Entrinsic Biosciences and Nocion Therapeutics, outside the submitted work. H.H. reports grants or contracts from Canon Medical Systems Inc and Konica-Minolta Inc; and consulting fees from Canon Medical Systems Inc and Mitsubishi Chemical Co, outside the submitted work. The other authors have no conflicts of interest to be disclosed related to this article.
Figures
References
-
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. (for the COVE Study Group) - DOI - PMC - PubMed
-
- Johnson A.G., Amin A.B., Ali A.R., Hoots B., Cadwell B.L., Arora S., Avoundjian T., Awofeso A.O., Barnes J., Bayoumi N.S., Busen K., Chang C., Cima M., Crockett M., Cronquist A., Davidson S., Davis E., Delgadillo J., Dorabawila V., Drenzek C., Eisenstein L., Fast H.E., Gent A., Hand J., Hoefer D., Holtzman C., Jara A., Jones A., Kamal-Ahmed I., Kangas S., Kanishka F., Kaur R., Khan S., King J., Kirkendall S., Klioueva A., Kocharian A., Kwon F.Y., Logan J., Lyons B.C., Lyons S., May A., McCormick D., Mshi E., Mendoza L., Milroy A., O’Donnell M., Pike S., Pogosjans A., Saupe J., Sell E., Smith D.M., Sosin E., Stanislawski M.K., Steele M., Stephenson A., Stout K., Strand B.P., Tilakaratne K., Turner H., Vest S., Warner C., Wiedeman A., Zaldivar B.J., Silk H.M., Scobie COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence – 25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022;71(4):132–138. doi: 10.15585/mmwr.mm7104e2. - DOI - PMC - PubMed
-
- Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., McNeal T., Ghamande S., Douin D.J., Talbot H.K., Casey J.D., Mohr N.M., Zepeski A., Shapiro N.I., Gibbs K.W., Files D.C., Hager D.N., Shehu A., Prekker M.E., Erickson H.L., Exline M.C., Gong M.N., Mohamed A., Johnson N.J., Srinivasan V., Steingrub J.S., Peltan I.D., Brown S.M., Martin E.T., Monto A.S., Khan A., Hough C.L., Busse L.W., Ten Lohuis C.C., Duggal A., Wilson J.G., Gordon A.J., Qadir N., Chang S.Y., Mallow C., Rivas C., Babcock H.M., Kwon J.H., Halasa N., Grijalva C.G., Rice T.W., Stubblefield W.B., Baughman A., Womack K.N., Rhoads J.P., Lindsell C.J., Hart K.W., Zhu Y., Adams K., Schrag S.J., Olson S.M., Kobayashi M., Verani J.R., Patel M.M., Self W.H. On behalf of Influenza and Other Viruses in the Acutely Ill (IVY) Network, Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069761. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

