Electrochemical reduction of nitrobenzene via redox-mediated chronoamperometry
- PMID: 36386882
- PMCID: PMC9641269
- DOI: 10.1016/j.xpro.2022.101817
Electrochemical reduction of nitrobenzene via redox-mediated chronoamperometry
Abstract
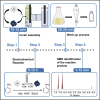
Anilines are important feedstocks for pharmaceuticals, dyes, and other materials, but traditional approaches to their syntheses usually lack selectivity and environmental sustainability. Here, we describe the selective reduction of nitrobenzene to aniline under mild conditions, using water as the ultimate source of the required protons and electrons. We describe the electrochemical cell assembly, and detail steps for electrochemical reduction followed by organic extraction and analysis of the extracts using NMR. For complete details on the use and execution of this protocol, please refer to Stergiou and Symes (2022a).
Keywords: Chemistry; Material sciences; NMR.
© 2022 The Author(s).
Conflict of interest statement
A.D.S. and M.D.S. have filed a patent related to this work (EP22386046.1.).
Figures







References
-
- Baumeister P., Blaser H.U., Scherrer W. Chemoselective hydrogenation of aromatic chloronitro compounds with amidine modified nickel catalysts. Stud. Surf. Sci. Catal. 1991;59:321–328. doi: 10.1016/S0167-2991(08)61137-4. - DOI
-
- Daems N., Risplendi F., Baert K., Hubin A., Vankelecom I.F.J., Cicero G., Pescarmona P.P. Doped ordered mesoporous carbons as novel, selective electrocatalysts for the reduction of nitrobenzene to aniline. J. Mater. Chem. A Mater. 2018;6:13397–13411. doi: 10.1039/C8TA01609G. - DOI
-
- Doherty S., Knight J.G., Backhouse T., Summers R.J., Abood E., Simpson W., Paget W., Bourne R.A., Chamberlain T.W., Stones R., et al. Highly selective and solvent dependent reduction of nitrobenzene to N-phenylhydroxylamine, azoxybenzene, and aniline catalyzed by phosphino-modified polymer immobilized ionic liquid-stabilized AuNPs. ACS Catal. 2019;9:4777–4791. doi: 10.1021/acscatal.9b00347. - DOI
-
- Ellis F. Royal Society of Chemistry; 2002. Paracetamol: A Curriculum Resource.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

