Glutathione: A Samsonian life-sustaining small molecule that protects against oxidative stress, ageing and damaging inflammation
- PMID: 36386929
- PMCID: PMC9664149
- DOI: 10.3389/fnut.2022.1007816
Glutathione: A Samsonian life-sustaining small molecule that protects against oxidative stress, ageing and damaging inflammation
Abstract
Many local and systemic diseases especially diseases that are leading causes of death globally like chronic obstructive pulmonary disease, atherosclerosis with ischemic heart disease and stroke, cancer and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 19 (COVID-19), involve both, (1) oxidative stress with excessive production of reactive oxygen species (ROS) that lower glutathione (GSH) levels, and (2) inflammation. The GSH tripeptide (γ- L-glutamyl-L-cysteinyl-glycine), the most abundant water-soluble non-protein thiol in the cell (1-10 mM) is fundamental for life by (a) sustaining the adequate redox cell signaling needed to maintain physiologic levels of oxidative stress fundamental to control life processes, and (b) limiting excessive oxidative stress that causes cell and tissue damage. GSH activity is facilitated by activation of the Kelch-like ECH-associated protein 1 (Keap1)-Nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element (ARE) redox regulator pathway, releasing Nrf2 that regulates expression of genes controlling antioxidant, inflammatory and immune system responses. GSH exists in the thiol-reduced (>98% of total GSH) and disulfide-oxidized (GSSG) forms, and the concentrations of GSH and GSSG and their molar ratio are indicators of the functionality of the cell. GSH depletion may play a central role in inflammatory diseases and COVID-19 pathophysiology, host immune response and disease severity and mortality. Therapies enhancing GSH could become a cornerstone to reduce severity and fatal outcomes of inflammatory diseases and COVID-19 and increasing GSH levels may prevent and subdue these diseases. The life value of GSH makes for a paramount research field in biology and medicine and may be key against systemic inflammation and SARS-CoV-2 infection and COVID-19 disease. In this review, we emphasize on (1) GSH depletion as a fundamental risk factor for diseases like chronic obstructive pulmonary disease and atherosclerosis (ischemic heart disease and stroke), (2) importance of oxidative stress and antioxidants in SARS-CoV-2 infection and COVID-19 disease, (3) significance of GSH to counteract persistent damaging inflammation, inflammaging and early (premature) inflammaging associated with cell and tissue damage caused by excessive oxidative stress and lack of adequate antioxidant defenses in younger individuals, and (4) new therapies that include antioxidant defenses restoration.
Keywords: COVID-19; atherosclerosis; chronic obstructive pulmonary disease; glutathione; inflammaging; nuclear factor erythroid 2-related factor 2; oxidative stress; reactive oxygen species.
Copyright © 2022 Labarrere and Kassab.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
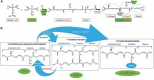



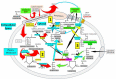


References
-
- Sastre J, Pallardo FV, Viña J. Glutathione. Handb Environ Chem. (2005) 2:91–108. 10.1007/b101148 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

