Fatal cases involving new psychoactive substances and trends in analytical techniques
- PMID: 36387045
- PMCID: PMC9640761
- DOI: 10.3389/ftox.2022.1033733
Fatal cases involving new psychoactive substances and trends in analytical techniques
Abstract
New psychoactive substances (NPS) are an emerging public health issue and deaths are commonly associated with polydrug abuse. Moreover, the number of new substances available is constantly increasing, causing intoxications in low doses, characteristics that impose to toxicology and forensic laboratories to keep routine methods up to date, with high detectability and constantly acquiring new analytical standards. Likewise, NPS metabolites and respective elimination pathways are usually unknown, making it difficult the detection and confirmation of the drug involved in the fatal case in an analytical routine. A literature search was performed on PubMed, Scopus and Web of Science databases for papers related to chromatographic analyses from fatal cases related to NPS use published from 2016 to 2021. A total of 96 papers were retrieved and reviewed in this study. Opioids, synthetic cathinones, phenethylamines/amphetamines and cannabinoids were the NPS classes most found in the fatal cases. In many cases, multiple compounds were detected in the biological samples, including prescription and other illegal drugs. Liquid chromatography-tandem mass spectrometry, an alternative to overcome the gas chromatography-mass spectrometry limitations for some compounds, was the analytical technique most used in the studies, and high resolution mass spectrometry was often applied to NPS metabolite investigation and structural characterization and identification of unknown compounds. Toxicological screening and quantitation methods need to be continuously updated to include new substances that are emerging on the drug market that can be fatal at very low doses.
Keywords: GC-MS; HRMS; LC-MS/MS; fatal cases; new psychoactive substances; opioids; synthetic cathinones.
Copyright © 2022 Ferrari Júnior, Leite, Gomes, Vieira, Sepulveda and Caldas.
Conflict of interest statement
TV was employed by Brainfarma Pharmaceutical Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

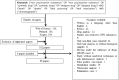
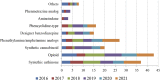
References
-
- Adamowicz P., Gieroń J., Gil D., Lechowicz W., Skulska A., Tokarczyk B., et al. (2016). Blood concentrations of α-pyrrolidinovalerophenone (α-PVP) determined in 66 forensic samples. Forensic Toxicol. 34, 227–234. 10.1007/s11419-016-0306-0 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

