Chemokine switch regulated by TGF-β1 in cancer-associated fibroblast subsets determines the efficacy of chemo-immunotherapy
- PMID: 36387055
- PMCID: PMC9662195
- DOI: 10.1080/2162402X.2022.2144669
Chemokine switch regulated by TGF-β1 in cancer-associated fibroblast subsets determines the efficacy of chemo-immunotherapy
Abstract
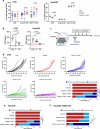
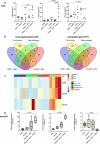
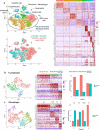
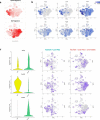
Combining immunogenic cell death-inducing chemotherapies and PD-1 blockade can generate remarkable tumor responses. It is now well established that TGF-β1 signaling is a major component of treatment resistance and contributes to the cancer-related immunosuppressive microenvironment. However, whether TGF-β1 remains an obstacle to immune checkpoint inhibitor efficacy when immunotherapy is combined with chemotherapy is still to be determined. Several syngeneic murine models were used to investigate the role of TGF-β1 neutralization on the combinations of immunogenic chemotherapy (FOLFOX: 5-fluorouracil and oxaliplatin) and anti-PD-1. Cancer-associated fibroblasts (CAF) and immune cells were isolated from CT26 and PancOH7 tumor-bearing mice treated with FOLFOX, anti-PD-1 ± anti-TGF-β1 for bulk and single cell RNA sequencing and characterization. We showed that TGF-β1 neutralization promotes the therapeutic efficacy of FOLFOX and anti-PD-1 combination and induces the recruitment of antigen-specific CD8+ T cells into the tumor. TGF-β1 neutralization is required in addition to chemo-immunotherapy to promote inflammatory CAF infiltration, a chemokine production switch in CAF leading to decreased CXCL14 and increased CXCL9/10 production and subsequent antigen-specific T cell recruitment. The immune-suppressive effect of TGF-β1 involves an epigenetic mechanism with chromatin remodeling of CXCL9 and CXCL10 promoters within CAF DNA in a G9a and EZH2-dependent fashion. Our results strengthen the role of TGF-β1 in the organization of a tumor microenvironment enriched in myofibroblasts where chromatin remodeling prevents CXCL9/10 production and limits the efficacy of chemo-immunotherapy.
Keywords: TGF-β; cancer; chemo-immunotherapy; chemokine; fibroblast; microenvironment.
© 2022 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
CB declares research grant from Roche, Bayer and advisory board for MSD, Sanofi, Bayer. All other authors report no conflict of interest.
Figures

References
-
- Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554(7693):538–543. doi:10.1038/nature25492. - DOI - PubMed
-
- de Streel G, Bertrand C, Chalon N, Liénart S, Bricard O, Lecomte S, Devreux J, Gaignage M, De Boeck G, Mariën L, et al. Selective inhibition of TGF-β1 produced by GARP-expressing Tregs overcomes resistance to PD-1/PD-L1 blockade in cancer. Nat Commun. 2020;11(1):4545. doi:10.1038/s41467-020-17811-3. - DOI - PMC - PubMed
-
- Strauss J, Heery CR, Schlom J, Madan RA, Cao L, Kang Z, Lamping E, Marté JL, Donahue RN, Grenga I, et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin Cancer Res. 2018;24(6):1287–1295. doi:10.1158/1078-0432.CCR-17-2653. - DOI - PMC - PubMed
-
- Kang Y-K, Bang Y-J, Kondo S, Chung HC, Muro K, Dussault I, Helwig C, Osada M, Doi T.. Safety and Tolerability of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFβ and PD-L1, in Asian Patients with Pretreated Recurrent or Refractory Gastric Cancer. Clinical Cancer Research. 2020;26(13):3202–3210. doi:10.1158/1078-0432.CCR-19-3806. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
