Updates in combined approaches of radiotherapy and immune checkpoint inhibitors for the treatment of breast cancer
- PMID: 36387071
- PMCID: PMC9643771
- DOI: 10.3389/fonc.2022.1022542
Updates in combined approaches of radiotherapy and immune checkpoint inhibitors for the treatment of breast cancer
Abstract
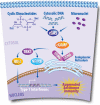
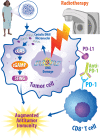
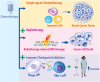
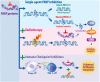
Breast cancer is the most prevalent non-skin cancer diagnosed in females and developing novel therapeutic strategies to improve patient outcomes is crucial. The immune system plays an integral role in the body's response to breast cancer and modulating this immune response through immunotherapy is a promising therapeutic option. Although immune checkpoint inhibitors were recently approved for the treatment of breast cancer patients, not all patients respond to immune checkpoint inhibitors as a monotherapy, highlighting the need to better understand the biology underlying patient response. Additionally, as radiotherapy is a critical component of breast cancer treatment, understanding the interplay of radiation and immune checkpoint inhibitors will be vital as recent studies suggest that combined therapies may induce synergistic effects in preclinical models of breast cancer. This review will discuss the mechanisms supporting combined approaches with radiotherapy and immune checkpoint inhibitors for the treatment of breast cancer. Moreover, this review will analyze the current clinical trials examining combined approaches of radiotherapy, immunotherapy, chemotherapy, and targeted therapy. Finally, this review will evaluate data regarding treatment tolerance and potential biomarkers for these emerging therapies aimed at improving breast cancer outcomes.
Keywords: breast cancer; immune checkpoint inhibitors (ICI); immunotherapy; radiation biology; radiotherapy; tumor immunology.
Copyright © 2022 Jungles, Holcomb, Pearson, Jungles, Bishop, Pierce, Green and Speers.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

