Case report: Immunovirotherapy as a novel add-on treatment in a patient with thoracic NUT carcinoma
- PMID: 36387105
- PMCID: PMC9647065
- DOI: 10.3389/fonc.2022.995744
Case report: Immunovirotherapy as a novel add-on treatment in a patient with thoracic NUT carcinoma
Abstract
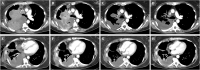
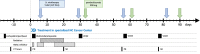
NUT carcinoma (NC) is a rare and extremely aggressive form of cancer, usually presenting with intrathoracic or neck manifestations in adolescents and young adults. With no established standard therapy regimen and a median overall survival of only 6.5 months, there is a huge need for innovative treatment options. As NC is genetically driven by a single aberrant fusion oncoprotein, it is generally characterized by a low tumor mutational burden, thus making it immunologically cold and insusceptible to conventional immunotherapy. Recently, we have demonstrated that oncolytic viruses (OVs) are able to specifically infect and lyse NC cells, thereby turning an immunologically cold tumor microenvironment into a hot one. Here, we report an intensive multimodal treatment approach employing for the first time an OV (talimogene laherparepvec (T-VEC); IMLYGIC®) together with the immune checkpoint inhibitor pembrolizumab as an add-on to a basic NC therapy (cytostatic chemotherapy, radiation therapy, epigenetic therapy) in a patient suffering from a large thoracic NC tumor which exhibits an aberrant, unique BRD3:NUTM1 fusion. This case demonstrates for the first time the feasibility of this innovative add-on immunovirotherapy regimen with a profound, repetitive and durable replication of T-VEC that is instrumental in achieving tumor stabilization and improvement in the patient´s quality of life. Further, a previously unknown BRD3:NUTM1 fusion gene was discovered that lacks the extraterminal domain of BRD3.
Keywords: BRD3; NUT carcinoma; NUTM1; T-VEC; chemotherapy; immunotherapy; virotherapy.
Copyright © 2022 Kloker, Calukovic, Benzler, Golf, Böhm, Günther, Horger, Haas, Berchtold, Beil, Carter, Ganzenmueller, Singer, Agaimy, Stöhr, Hartmann, Duell, Mairhofer, Fohrer, Reinmuth, Zender and Lauer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Multimodal Therapy Approaches for NUT Carcinoma by Dual Combination of Oncolytic Virus Talimogene Laherparepvec with Small Molecule Inhibitors.Viruses. 2024 May 14;16(5):775. doi: 10.3390/v16050775. Viruses. 2024. PMID: 38793657 Free PMC article.
-
Efficacy of Oncolytic Herpes Simplex Virus T-VEC Combined with BET Inhibitors as an Innovative Therapy Approach for NUT Carcinoma.Cancers (Basel). 2022 Jun 2;14(11):2761. doi: 10.3390/cancers14112761. Cancers (Basel). 2022. PMID: 35681742 Free PMC article.
-
BRD3-NUTM1-expressing NUT carcinoma of lung on endobronchial ultrasound-guided transbronchial needle aspiration cytology, a diagnostic pitfall.Diagn Cytopathol. 2022 Feb;50(2):E47-E53. doi: 10.1002/dc.24885. Epub 2021 Oct 21. Diagn Cytopathol. 2022. PMID: 34672128
-
Advances in the pathogenesis and treatment of nut carcinoma: a narrative review.Transl Cancer Res. 2020 Oct;9(10):6505-6515. doi: 10.21037/tcr-20-1884. Transl Cancer Res. 2020. PMID: 35117258 Free PMC article. Review.
-
New hopes for the breast cancer treatment: perspectives on the oncolytic virus therapy.Front Immunol. 2024 Mar 21;15:1375433. doi: 10.3389/fimmu.2024.1375433. eCollection 2024. Front Immunol. 2024. PMID: 38576614 Free PMC article. Review.
Cited by
-
Multimodal Therapy Approaches for NUT Carcinoma by Dual Combination of Oncolytic Virus Talimogene Laherparepvec with Small Molecule Inhibitors.Viruses. 2024 May 14;16(5):775. doi: 10.3390/v16050775. Viruses. 2024. PMID: 38793657 Free PMC article.
-
Adding checkpoint inhibitors to first-line chemotherapy for NUT carcinoma patients.NPJ Precis Oncol. 2025 Jan 25;9(1):26. doi: 10.1038/s41698-024-00768-7. NPJ Precis Oncol. 2025. PMID: 39863772 Free PMC article.
-
Anti-angiogenic therapy as a beacon of hope in the battle against pulmonary NUT midline carcinoma.Front Med. 2025 Aug;19(4):681-688. doi: 10.1007/s11684-025-1145-3. Epub 2025 Jun 14. Front Med. 2025. PMID: 40522617
-
Hiding in plain sight: NUT carcinoma is an unrecognized subtype of squamous cell carcinoma of the lungs and head and neck.Nat Rev Clin Oncol. 2025 Apr;22(4):292-306. doi: 10.1038/s41571-025-00986-3. Epub 2025 Feb 3. Nat Rev Clin Oncol. 2025. PMID: 39900969 Review.
-
Case report: NUT carcinoma with MXI1::NUTM1 fusion characterized by abdominopelvic lesions and ovarian masses in a middle-aged female.Front Oncol. 2023 Jan 11;12:1091877. doi: 10.3389/fonc.2022.1091877. eCollection 2022. Front Oncol. 2023. PMID: 36741693 Free PMC article.
References
-
- Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R, et al. . An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: Analysis of 124 patients. JNCI Cancer Spectr (2020) 4(2):pkz094. doi: 10.1093/jncics/pkz094 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

