Mono- and biallelic germline variants of DNA glycosylase genes in colon adenomatous polyposis families from two continents
- PMID: 36387175
- PMCID: PMC9650540
- DOI: 10.3389/fonc.2022.870863
Mono- and biallelic germline variants of DNA glycosylase genes in colon adenomatous polyposis families from two continents
Abstract
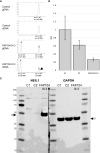
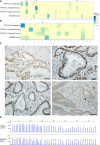
Recently, biallelic germline variants of the DNA glycosylase genes MUTYH and NTHL1 were linked to polyposis susceptibility. Significant fractions remain without a molecular explanation, warranting searches for underlying causes. We used exome sequencing to investigate clinically well-defined adenomatous polyposis cases and families from Finland (N=34), Chile (N=21), and Argentina (N=12), all with known susceptibility genes excluded. Nine index cases (13%) revealed germline variants with proven or possible pathogenicity in the DNA glycosylase genes, involving NEIL1 (mono- or biallelic) in 3 cases, MUTYH (monoallelic) in 3 cases, NTHL1 (biallelic) in 1 case, and OGG1 (monoallelic) in 2 cases. NTHL1 was affected with the well-established, pathogenic c.268C>T, p.(Gln90Ter) variant. A recurrent heterozygous NEIL1 c.506G>A, p.(Gly169Asp) variant was observed in two families. In a Finnish family, the variant occurred in trans with a truncating NEIL1 variant (c.821delT). In an Argentine family, the variant co-occurred with a genomic deletion of exons 2 - 11 of PMS2. Mutational signatures in tumor tissues complied with biological functions reported for NEIL1. Our results suggest that germline variants in DNA glycosylase genes may occur in a non-negligible proportion of unexplained colon polyposis cases and may predispose to tumor development.
Keywords: DNA glycosylase; MUTYH; NEIL1; NTHL1; OGG1; exome sequencing; germline variant; polyposis.
Copyright © 2022 Olkinuora, Mayordomo, Kauppinen, Cerliani, Coraglio, Collia, Gutiérrez, Alvarez, Cassana, Lopéz-Köstner, Jauk, García-Rivello, Ristimäki, Koskenvuo, Lepistö, Nieminen, Vaccaro, Pavicic and Peltomäki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

