Fourteen-Day Evolution of COVID-19 Symptoms during the Third Wave in Nonvaccinated Subjects and Effects of Hesperidin Therapy: A Randomized, Double-Blinded, Placebo-Controlled Study
- PMID: 36387348
- PMCID: PMC9649310
- DOI: 10.1155/2022/3125662
Fourteen-Day Evolution of COVID-19 Symptoms during the Third Wave in Nonvaccinated Subjects and Effects of Hesperidin Therapy: A Randomized, Double-Blinded, Placebo-Controlled Study
Abstract
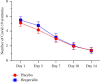
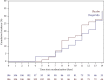
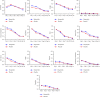
COVID-19 symptoms can cause substantial disability, yet no therapy can currently reduce their frequency or duration. We conducted a double-blind placebo-controlled trial of hesperidin 1000 mg once daily for 14 days in 216 symptomatic nonvaccinated COVID-19 subjects. Thirteen symptoms were recorded after 3, 7, 10, and 14 days. The primary endpoint was the proportion of subjects with any of four cardinal (group A) symptoms: fever, cough, shortness of breath, or anosmia. At the baseline, symptoms in decreasing frequency were as follows: cough (53.2%), weakness (44.9%), headache (42.6%), pain (35.2%), sore throat (28.7%), runny nose (26.9%), chills (22.7%), shortness of breath (22.2%), anosmia (18.5%), fever (16.2%), diarrhea (6.9%), nausea/vomiting (6.5%), and irritability/confusion (3.2%). Group A symptoms in the placebo vs. hesperidin group were 88.8% vs. 88.5% (day 1) and reduced to 58.5 vs. 49.4% at day 14 (OR 0.69, 95% CI 0.38-1.27, p = 0.23). At day 14, 15 subjects in the placebo group and 28 in the hesperidin group failed to report their symptoms. In an attrition bias analysis imputing "no symptoms" to missing values, the hesperidin group showed reduction of 14.5% of group A symptoms from 50.9% to 36.4% (OR: 0.55, 0.32-0.96, p = 0.03). Anosmia, the most frequent persisting symptom (29.3%), was lowered by 7.3% to 25.3% in the hesperidin group vs. 32.6% in the placebo group (p = 0.29). The mean number of symptoms in the placebo and hesperidin groups was 5.10 (SD 2.26) vs. 5.48 (SD 2.35) (day 1) and 1.40 (SD 1.65) vs. 1.38 (SD 1.76) (day 14) (p = 0.92). In conclusion, most nonvaccinated COVID-19 infected subjects remain symptomatic after 14 days with anosmia being the most frequently persisting symptom. Hesperidin 1 g daily may help reduce group A symptoms. Earlier treatment of longer duration and/or higher dosage should be tested.
Copyright © 2022 Jocelyn Dupuis et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial.EClinicalMedicine. 2023 Dec 14;67:102359. doi: 10.1016/j.eclinm.2023.102359. eCollection 2024 Jan. EClinicalMedicine. 2023. PMID: 38188690 Free PMC article.
-
Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):343. doi: 10.1186/s13063-021-05181-0. Trials. 2021. PMID: 34001215 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2021 Feb 23;2(2):CD013665. doi: 10.1002/14651858.CD013665.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 20;5:CD013665. doi: 10.1002/14651858.CD013665.pub3. PMID: 33620086 Free PMC article. Updated.
-
Interventions for reducing inflammation in familial Mediterranean fever.Cochrane Database Syst Rev. 2018 Oct 19;10(10):CD010893. doi: 10.1002/14651858.CD010893.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Mar 29;3:CD010893. doi: 10.1002/14651858.CD010893.pub4. PMID: 30338514 Free PMC article. Updated.
Cited by
-
Preventing the Adverse Effects of SARS-CoV-2 Infection and COVID-19 through Diet, Supplements, and Lifestyle.Nutrients. 2021 Dec 28;14(1):115. doi: 10.3390/nu14010115. Nutrients. 2021. PMID: 35010990 Free PMC article.
-
Post-COVID-19 Anosmia and Therapies: Stay Tuned for New Drugs to Sniff Out.Diseases. 2023 May 27;11(2):79. doi: 10.3390/diseases11020079. Diseases. 2023. PMID: 37366867 Free PMC article. Review.
-
Plausibility of natural immunomodulators in the treatment of COVID-19-A comprehensive analysis and future recommendations.Heliyon. 2023 Jun;9(6):e17478. doi: 10.1016/j.heliyon.2023.e17478. Epub 2023 Jun 21. Heliyon. 2023. PMID: 37366526 Free PMC article. Review.
-
Hesperidin, a Potential Antiviral Agent against SARS-CoV-2: The Influence of Citrus Consumption on COVID-19 Incidence and Severity in China.Medicina (Kaunas). 2024 May 28;60(6):892. doi: 10.3390/medicina60060892. Medicina (Kaunas). 2024. PMID: 38929512 Free PMC article. Review.
-
Bioinformatics-based investigation on the genetic influence between SARS-CoV-2 infections and idiopathic pulmonary fibrosis (IPF) diseases, and drug repurposing.Sci Rep. 2023 Mar 22;13(1):4685. doi: 10.1038/s41598-023-31276-6. Sci Rep. 2023. PMID: 36949176 Free PMC article.
References
-
- Beltran-Corbellini A., Chico-Garcia J. L., Martinez-Poles J., et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. European Journal of Neurology . 2020;27(9):1738–1741. doi: 10.1111/ene.14273. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

