Increasing the effect of annonacin using nanodiamonds to inhibit breast cancer cells growth in rats (Rattus norvegicus)-Induced breast cancer
- PMID: 36387488
- PMCID: PMC9650002
- DOI: 10.1016/j.heliyon.2022.e11418
Increasing the effect of annonacin using nanodiamonds to inhibit breast cancer cells growth in rats (Rattus norvegicus)-Induced breast cancer
Abstract
Background: Annonaceous acetogenins have been reported to have anti-cancer properties but low viability. In this study, we aimed to investigate the potency of nanodiamonds to be employed as a carrier of annonacin to help increase its viability and inhibit the growth of breast cancer cells.
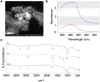
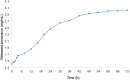
Methods: The annonacin was coupled with nanodiamond and characterized using UV-Vis spectrophotometer, FTIR, SEM, and PSA, and determined their stability and drug release. A cell growth inhibition assay and cell migration assay was performed using the breast cancer MCF7 and T747D cell lines, and in vivo analysis was performed in rats (Rattus norvegicus). MCF7 and T747D cells were treated with 12.5 μg/mL annonacin coupled with nanodiamonds for 24 and 48 h and further analyzed by MTT, cell migration, and reactive oxygen species (ROS) assays. Twenty-five female rats were divided into five groups. Breast cancer was induced using two intraperitoneal doses of N-nitroso-N-methylurea (NMU) (50 and 30 mg/kg body weight). Annonacin coupled with nanodiamonds was administered by intraperitoneal injection (17.5 mg/kg body weight) for 5 weeks, one injection per 3 days.
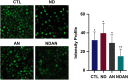
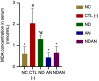
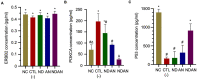
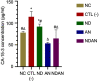
Results: Administration of annonacin coupled with nanodiamonds significantly reduced MCF7 cell growth and reactive oxygen species (ROS) levels. The in vivo study showed that administration of annonacin coupled with nanodiamonds significantly reduced PI3KCA levels and increased p53 expression, reduced cancer antigen-15-3 (CA-15-3) levels in serum, increased caspase-3 expression, reduced Ki-67 levels, and reduced the thickness of the mammary ductal epithelium.
Conclusions: Collectively, this study demonstrated the effectiveness of nanodiamonds as a carrier of annonacin to inhibit breast cancer cell growth through inhibition of the PI3K/Akt signaling pathway.
Keywords: Annonacin; Breast cancer; Nanodiamonds; PI3K; ROS.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Chloroform Fraction of Methanolic Extract of Seeds of Annona muricata Induce S Phase Arrest and ROS Dependent Caspase Activated Mitochondria-Mediated Apoptosis in Triple-Negative Breast Cancer.Anticancer Agents Med Chem. 2021;21(10):1250-1265. doi: 10.2174/1871520620666200918101448. Anticancer Agents Med Chem. 2021. PMID: 32951586
-
Annonacin Exerts Antitumor Activity through Induction of Apoptosis and Extracellular Signal-regulated Kinase Inhibition.Pharmacognosy Res. 2017 Oct-Dec;9(4):378-383. doi: 10.4103/pr.pr_19_17. Pharmacognosy Res. 2017. PMID: 29263632 Free PMC article.
-
Annonacin induces cell cycle-dependent growth arrest and apoptosis in estrogen receptor-α-related pathways in MCF-7 cells.J Ethnopharmacol. 2011 Oct 11;137(3):1283-90. doi: 10.1016/j.jep.2011.07.056. Epub 2011 Aug 4. J Ethnopharmacol. 2011. PMID: 21840388
-
Modulation of cancer signalling pathway(s) in two -stage mouse skin tumorigenesis by annonacin.BMC Complement Altern Med. 2019 Sep 3;19(1):238. doi: 10.1186/s12906-019-2650-1. BMC Complement Altern Med. 2019. PMID: 31481122 Free PMC article.
-
Nanodiamonds and their potential applications in breast cancer therapy: a narrative review.Drug Deliv Transl Res. 2022 May;12(5):1017-1028. doi: 10.1007/s13346-021-00996-5. Epub 2021 May 10. Drug Deliv Transl Res. 2022. PMID: 33970463 Review.
Cited by
-
Vaccinia-related kinase 2 variants differentially affect breast cancer growth by regulating kinase activity.Oncol Res. 2023 Dec 28;32(2):421-432. doi: 10.32604/or.2023.031031. eCollection 2023. Oncol Res. 2023. PMID: 38186576 Free PMC article.
-
Ethanolic Extract of Red Okra Pods Induces Aberrant Spindle Segregation and Apoptotic Cell Death by Disrupting the Wnt Signaling Pathway in Colon Cancer Cells.Iran J Med Sci. 2024 Dec 1;49(12):785-793. doi: 10.30476/IJMS.2024.99450.3149. eCollection 2024 Dec. Iran J Med Sci. 2024. PMID: 39840302 Free PMC article.
References
-
- Abraham A.G., O’Neill E. PI3K/Akt-mediated regulation of p53 in cancer. Biochem. Soc. Trans. 2014;42:798–803. - PubMed
-
- Burdick A.D., Davis J.W., Liu K.J., Hudson L.G., Shi H., Monske M.L., Burchiel S.W. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003;63:7825–7833. - PubMed
-
- Chan W.J., McLachlan A.J., Hanrahan J.R., et al. The safety and tolerability of Annona muricata leaf extract: a systematic review. J. Pharm. Pharmacol. 2019;72:1–16. - PubMed
-
- Chang J., Ormerod M., Powles T.J., Allred D.C., Ashley S.E., Dowsett M. Apoptosis and proliferation as predictors of chemotherapy response in patients with breast carcinoma. Cancer. 2000;89:2145–2152. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

