Differential effects of Akkermansia-enriched fecal microbiota transplant on energy balance in female mice on high-fat diet
- PMID: 36387852
- PMCID: PMC9647077
- DOI: 10.3389/fendo.2022.1010806
Differential effects of Akkermansia-enriched fecal microbiota transplant on energy balance in female mice on high-fat diet
Abstract
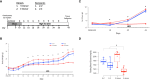
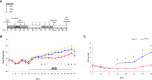
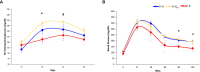
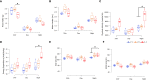
Estrogens protect against weight gain and metabolic disruption in women and female rodents. Aberrations in the gut microbiota composition are linked to obesity and metabolic disorders. Furthermore, estrogen-mediated protection against diet-induced metabolic disruption is associated with modifications in gut microbiota. In this study, we tested if estradiol (E2)-mediated protection against obesity and metabolic disorders in female mice is dependent on gut microbiota. Specifically, we tested if fecal microbiota transplantation (FMT) from E2-treated lean female mice, supplemented with or without Akkermansia muciniphila, prevented high fat diet (HFD)-induced body weight gain, fat mass gain, and hyperglycemia in female recipients. FMT from, and cohousing with, E2-treated lean donors was not sufficient to transfer the metabolic benefits to the E2-deficient female recipients. Moreover, FMT from lean donors supplemented with A. muciniphila exacerbated HFD-induced hyperglycemia in E2-deficient recipients, suggesting its detrimental effect on the metabolic health of E2-deficient female rodents fed a HFD. Given that A. muciniphila attenuates HFD-induced metabolic insults in males, the present findings suggest a sex difference in the impact of this microbe on metabolic health.
Keywords: diabetes; estradiol; estrogens; gut microbiome; metabolism; obesity.
Copyright © 2022 Acharya, Friedline, Ward, Graham, Tauer, Zheng, Hu, de Vos, McCormick, Kim and Tetel.
Conflict of interest statement
WV is co-founder and has stock in The Akkermansia Company, and BM is a coinventor on a patent application PGT/US 18/42116 emanating, in part, from the findings described herein, and along with her respective academic institution, stands to gain financially through potential commercialization outcomes resulting from activities associated with the licensing of that intellectual property. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Schmiegelow MD, Hedlin H, Mackey RH, Martin LW, Vitolins MZ, Stefanick ML, et al. . Race and ethnicity, obesity, metabolic health, and risk of cardiovascular disease in postmenopausal women. J Am Heart Association: Cardiovasc Cerebrovascular Dis (2015) 4(5). doi: 10.1161/JAHA.114.001695 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

