Stereology of gonadotropin-releasing hormone and kisspeptin neurons in PACAP gene-deficient female mice
- PMID: 36387875
- PMCID: PMC9640735
- DOI: 10.3389/fendo.2022.993228
Stereology of gonadotropin-releasing hormone and kisspeptin neurons in PACAP gene-deficient female mice
Abstract
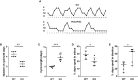
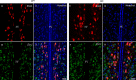
The hypothalamic gonadotropin-releasing hormone (GnRH)-kisspeptin neuronal network regulates fertility in all mammals. Pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide isolated from the hypothalamus that is involved in the regulation of several releasing hormones and trop hormones. It is well-known that PACAP influences fertility at central and peripheral levels. However, the effects of PACAP on GnRH and kisspeptin neurons are not well understood. The present study investigated the integrity of the estrous cycle in PACAP-knockout (KO) mice. The number and immunoreactivity of GnRH (GnRH-ir) neurons in wild-type (WT) and PACAP KO female mice were determined using immunohistochemistry. In addition, the number of kisspeptin neurons was measured by counting kisspeptin mRNA-positive cells in the rostral periventricular region of the third ventricle (RP3V) and arcuate nucleus (ARC) using the RNAscope technique. Finally, the mRNA and protein expression of estrogen receptor alpha (ERα) was also examined. Our data showed that the number of complete cycles decreased, and the length of each cycle was longer in PACAP KO mice. Furthermore, the PACAP KO mice experienced longer periods of diestrus and spent significantly less time in estrus. There was no difference in GnRH-ir or number of GnRH neurons. In contrast, the number of kisspeptin neurons was decreased in the ARC, but not in the R3PV, in PACAP KO mice compared to WT littermates. Furthermore, ERα mRNA and protein expression was decreased in the ARC, whereas in the R3PV region, ERα mRNA levels were elevated. Our results demonstrate that embryonic deletion of PACAP significantly changes the structure and presumably the function of the GnRH-kisspeptin neuronal network, influencing fertility.
Keywords: GnRH; PACAP; estrogen receptor α; estrous cycle; kisspeptin.
Copyright © 2022 Barabás, Kovács, Vértes, Kövesdi, Faludi, Udvarácz, Pham, Reglődi, Abraham and Nagy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

