A pilot investigation of the efficacy and safety of magnesium chloride and ethanol as anesthetics in Loligo vulgaris embryos
- PMID: 36388114
- PMCID: PMC9641376
- DOI: 10.3389/fphys.2022.968047
A pilot investigation of the efficacy and safety of magnesium chloride and ethanol as anesthetics in Loligo vulgaris embryos
Abstract
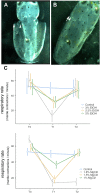
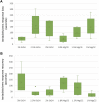
The inclusion of cephalopods in the legislation related to the use of animals for experimental purposes has been based on the precautionary principle that these animals have the capacity to experience pain, suffering, distress, and lasting harm. Recent studies have expanded this view and supported it. Handling cephalopod mollusks in research is challenging and whenever more invasive procedures are required, sedation and/or anesthesia becomes necessary. Therefore, finding adequate, safe, and effective anesthetics appears mandatory. Several substances have been considered in sedating cephalopods, in some instances applying those utilized for fish. However, species-specific variability requires more detailed studies. Despite long-lasting experience being linked to classic studies on squid giant axons, evidence of action on putative anesthetic substances is scarce for Loligo vulgaris and particularly for their embryos. The aim of the current study was to evaluate effects elicited by immersion of squid embryos in anesthetic solutions and examine whether these forms display a similar reaction to anesthetics as adults do. Different concentrations of ethanol (EtOH; 2, 2.5, and 3%) and magnesium chloride (MgCl2; 1, 1.5, and 1.8%) were tested by adopting a set of indicators aimed at exploring the physiological responses of squid embryos. Forty-two embryos of the common squid Loligo vulgaris (stages 27-28) were assigned to three conditions (EtOH, MgCl2, and controls) and video recorded for 15 min (5 min before, 5 min during, and 5 min after immersion in the anesthetic solutions). In each group, the heart rate, respiratory rate, buoyancy, chromatophore activity, and tentacles/arms responses were assessed to evaluate the embryos' vitality and responsiveness to stimulation. Both substances provoked a decrease in heart and respiratory rates and inhibited buoyancy, chromatophores, and tentacles/arms responses; no adverse effects were observed. EtOH had a faster onset of action and faster recovery than MgCl2, being potentially more adequate as an anesthetic for shorter procedures. Even though MgCl2 caused a longer muscle relaxation, the reversibility was not confirmed for the 1.8% concentration; however, lower concentrations triggered similar results as the ones obtained with the highest EtOH concentrations. We have shown that the late developmental stages of Loligo vulgaris embryos could represent a good model to evaluate anesthetics for cephalopods since they can display similar reactions to anesthetics as adults animals do.
Keywords: Loligo vulgaris; administration and dosage; anesthesia; cephalopods embryos; ethanol; magnesium chloride (MgCl2).
Copyright © 2022 Sprecher, Sprecher and Spadavecchia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Effect of Different Formulations of Magnesium Chloride Used As Anesthetic Agents on the Performance of the Isolated Heart of Octopus vulgaris.Front Physiol. 2016 Dec 26;7:610. doi: 10.3389/fphys.2016.00610. eCollection 2016. Front Physiol. 2016. PMID: 28082904 Free PMC article.
-
Molecular characterization of cell types in the squid Loligo vulgaris.Elife. 2023 Jan 3;12:e80670. doi: 10.7554/eLife.80670. Elife. 2023. PMID: 36594460 Free PMC article.
-
Short and Long-Term Effects of Anesthesia in Octopus maya (Cephalopoda, Octopodidae) Juveniles.Front Physiol. 2020 Jun 30;11:697. doi: 10.3389/fphys.2020.00697. eCollection 2020. Front Physiol. 2020. PMID: 32695019 Free PMC article.
-
Sense and Insensibility - An Appraisal of the Effects of Clinical Anesthetics on Gastropod and Cephalopod Molluscs as a Step to Improved Welfare of Cephalopods.Front Physiol. 2018 Aug 24;9:1147. doi: 10.3389/fphys.2018.01147. eCollection 2018. Front Physiol. 2018. PMID: 30197598 Free PMC article. Review.
-
MgCl2 and its applications in organic chemistry and biochemistry: a review.Mol Divers. 2020 May;24(2):463-476. doi: 10.1007/s11030-019-09947-2. Epub 2019 Apr 12. Mol Divers. 2020. PMID: 30980342 Review.
Cited by
-
Evaluation of Candidates for Systemic Analgesia and General Anesthesia in the Emerging Model Cephalopod, Euprymna berryi.Biology (Basel). 2023 Jan 28;12(2):201. doi: 10.3390/biology12020201. Biology (Basel). 2023. PMID: 36829480 Free PMC article.
References
-
- Andrews P. L. R., Darmaillacq A. S., Dennison N., Gleadall I. G., Hawkins P., Messenger J. B., et al. (2013). The identification and management of pain, suffering and distress in cephalopods, including anaesthesia, analgesia and humane killing. J. Exp. Mar. Biol. Ecol. 447, 46–64. 10.1016/j.jembe.2013.02.010 - DOI
-
- Arnold J. M., Summers W. C., Gilbert D. L., Manalis R. S., Daw N. W., Lasek R. J. (1974). A guide to laboratory use of the squid Loligo pealei. Woods Hole. Mass: Marine Biological Laboratory.
-
- Baldascino E., Di Cristina G., Tedesco P., Hobbs C., Shaw T. J., Ponte G., et al. (2017). The gastric ganglion of Octopus vulgaris: Preliminary characterization of gene- and putative neurochemical-complexity, and the effect of Aggregata octopiana digestive tract infection on gene expression. Front. Physiol. 8 (1001). 10.3389/fphys.2017.01001 - DOI - PMC - PubMed

