Stable potassium isotopes (41K/39K) track transcellular and paracellular potassium transport in biological systems
- PMID: 36388124
- PMCID: PMC9644202
- DOI: 10.3389/fphys.2022.1016242
Stable potassium isotopes (41K/39K) track transcellular and paracellular potassium transport in biological systems
Abstract
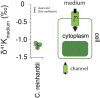
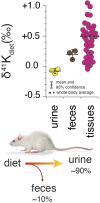
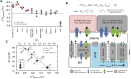
As the most abundant cation in archaeal, bacterial, and eukaryotic cells, potassium (K+) is an essential element for life. While much is known about the machinery of transcellular and paracellular K transport-channels, pumps, co-transporters, and tight-junction proteins-many quantitative aspects of K homeostasis in biological systems remain poorly constrained. Here we present measurements of the stable isotope ratios of potassium (41K/39K) in three biological systems (algae, fish, and mammals). When considered in the context of our current understanding of plausible mechanisms of K isotope fractionation and K+ transport in these biological systems, our results provide evidence that the fractionation of K isotopes depends on transport pathway and transmembrane transport machinery. Specifically, we find that passive transport of K+ down its electrochemical potential through channels and pores in tight-junctions at favors 39K, a result which we attribute to a kinetic isotope effect associated with dehydration and/or size selectivity at the channel/pore entrance. In contrast, we find that transport of K+ against its electrochemical gradient via pumps and co-transporters is associated with less/no isotopic fractionation, a result that we attribute to small equilibrium isotope effects that are expressed in pumps/co-transporters due to their slower turnover rate and the relatively long residence time of K+ in the ion pocket. These results indicate that stable K isotopes may be able to provide quantitative constraints on transporter-specific K+ fluxes (e.g., the fraction of K efflux from a tissue by channels vs. co-transporters) and how these fluxes change in different physiological states. In addition, precise determination of K isotope effects associated with K+ transport via channels, pumps, and co-transporters may provide unique constraints on the mechanisms of K transport that could be tested with steered molecular dynamic simulations.
Keywords: Na-K ATPase; homeostasis; physiology; potassium; potassium channel; stable isotope.
Copyright © 2022 Higgins, Ramos, Gili, Spetea, Kanoski, Ha, McDonough and Youn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Boron W. F., Boulpaep E. L. (2005). Medical physiology. Philadelphia, Pennsylvania: Elsevier Saunders.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

