Dexmedetomidine-mediated sleep phase modulation ameliorates motor and cognitive performance in a chronic blast-injured mouse model
- PMID: 36388181
- PMCID: PMC9663850
- DOI: 10.3389/fneur.2022.1040975
Dexmedetomidine-mediated sleep phase modulation ameliorates motor and cognitive performance in a chronic blast-injured mouse model
Abstract
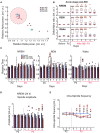
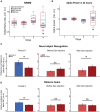
Multiple studies have shown that blast injury is followed by sleep disruption linked to functional sequelae. It is well established that improving sleep ameliorates such functional deficits. However, little is known about longitudinal brain activity changes after blast injury. In addition, the effects of directly modulating the sleep/wake cycle on learning task performance after blast injury remain unclear. We hypothesized that modulation of the sleep phase cycle in our injured mice would improve post-injury task performance. Here, we have demonstrated that excessive sleep electroencephalographic (EEG) patterns are accompanied by prominent motor and cognitive impairment during acute stage after secondary blast injury (SBI) in a mouse model. Over time we observed a transition to more moderate and prolonged sleep/wake cycle disturbances, including changes in theta and alpha power. However, persistent disruptions of the non-rapid eye movement (NREM) spindle amplitude and intra-spindle frequency were associated with lasting motor and cognitive deficits. We, therefore, modulated the sleep phase of injured mice using subcutaneous (SC) dexmedetomidine (Dex), a common, clinically used sedative. Dex acutely improved intra-spindle frequency, theta and alpha power, and motor task execution in chronically injured mice. Moreover, dexmedetomidine ameliorated cognitive deficits a week after injection. Our results suggest that SC Dex might potentially improve impaired motor and cognitive behavior during daily tasks in patients that are chronically impaired by blast-induced injuries.
Keywords: blast injury model; chronic blast injury; cognitive behavior; dexmedetomidine; motor behavior; sleep/wake cycle disturbance.
Copyright © 2022 Bibineyshvili, Schiff and Calderon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Oral Delivered Dexmedetomidine Promotes and Consolidates Non-rapid Eye Movement Sleep via Sleep-Wake Regulation Systems in Mice.Front Pharmacol. 2018 Dec 5;9:1196. doi: 10.3389/fphar.2018.01196. eCollection 2018. Front Pharmacol. 2018. PMID: 30568589 Free PMC article.
-
Alterations in sleep, sleep spindle, and EEG power in mGluR5 knockout mice.J Neurophysiol. 2020 Jan 1;123(1):22-33. doi: 10.1152/jn.00532.2019. Epub 2019 Nov 20. J Neurophysiol. 2020. PMID: 31747354 Free PMC article.
-
Structural and biochemical abnormalities in the absence of acute deficits in mild primary blast-induced head trauma.J Neurosurg. 2016 Mar;124(3):675-86. doi: 10.3171/2015.1.JNS141571. Epub 2015 Aug 21. J Neurosurg. 2016. PMID: 26295915 Free PMC article.
-
Wake and Sleep EEG in Patients With Huntington Disease: An eLORETA Study and Review of the Literature.Clin EEG Neurosci. 2017 Jan;48(1):60-71. doi: 10.1177/1550059416632413. Epub 2016 Apr 19. Clin EEG Neurosci. 2017. PMID: 27094758 Review.
-
Sleep and wake disturbances following traumatic brain injury.Pathol Biol (Paris). 2014 Oct;62(5):252-61. doi: 10.1016/j.patbio.2014.05.014. Epub 2014 Aug 7. Pathol Biol (Paris). 2014. PMID: 25110283 Review.
Cited by
-
Sleep, inflammation, and hemodynamics in rodent models of traumatic brain injury.Front Neurosci. 2024 Feb 15;18:1361014. doi: 10.3389/fnins.2024.1361014. eCollection 2024. Front Neurosci. 2024. PMID: 38426017 Free PMC article. Review.
-
Cortical signatures linked to behavior quantitatively track arousal levels.Proc Natl Acad Sci U S A. 2025 May 13;122(19):e2413789122. doi: 10.1073/pnas.2413789122. Epub 2025 May 5. Proc Natl Acad Sci U S A. 2025. PMID: 40324087
References
LinkOut - more resources
Full Text Sources
Research Materials

