A novel small open reading frame gene, IbEGF, enhances drought tolerance in transgenic sweet potato
- PMID: 36388596
- PMCID: PMC9660231
- DOI: 10.3389/fpls.2022.965069
A novel small open reading frame gene, IbEGF, enhances drought tolerance in transgenic sweet potato
Abstract
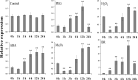
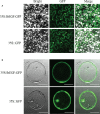
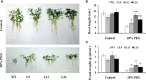
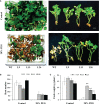
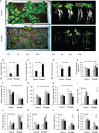
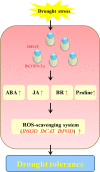
Small open reading frames (sORFs) can encode functional polypeptides or act as cis-translational regulators in stress responses in eukaryotes. Their number and potential importance have only recently become clear in plants. In this study, we identified a novel sORF gene in sweet potato, IbEGF, which encoded the 83-amino acid polypeptide containing an EGF_CA domain. The expression of IbEGF was induced by PEG6000, H2O2, abscisic acid (ABA), methyl-jasmonate (MeJA) and brassinosteroid (BR). The IbEGF protein was localized to the nucleus and cell membrane. Under drought stress, overexpression of IbEGF enhanced drought tolerance, promoted the accumulation of ABA, MeJA, BR and proline and upregulated the genes encoding superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) in transgenic sweet potato. The IbEGF protein was found to interact with IbCOP9-5α, a regulator in the phytohormone signalling pathways. These results suggest that IbEGF interacting with IbCOP9-5α enhances drought tolerance by regulating phytohormone signalling pathways, increasing proline accumulation and further activating reactive oxygen species (ROS) scavenging system in transgenic sweet potato.
Keywords: IbEGF; drought tolerance; phytohormone; sORF; sweet potato.
Copyright © 2022 Zhou, Zhai, Xing, Wei, He, Zhang, Gao, Zhao and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Expression of the Sweet Potato MYB Transcription Factor IbMYB48 Confers Salt and Drought Tolerance in Arabidopsis.Genes (Basel). 2022 Oct 17;13(10):1883. doi: 10.3390/genes13101883. Genes (Basel). 2022. PMID: 36292768 Free PMC article.
-
A Novel WRKY Transcription Factor from Ipomoea trifida, ItfWRKY70, Confers Drought Tolerance in Sweet Potato.Int J Mol Sci. 2022 Jan 8;23(2):686. doi: 10.3390/ijms23020686. Int J Mol Sci. 2022. PMID: 35054868 Free PMC article.
-
A C2-Domain Abscisic Acid-Related Gene, IbCAR1, Positively Enhances Salt Tolerance in Sweet Potato (Ipomoea batatas (L.) Lam.).Int J Mol Sci. 2022 Aug 26;23(17):9680. doi: 10.3390/ijms23179680. Int J Mol Sci. 2022. PMID: 36077077 Free PMC article.
-
A novel Cys2/His2 zinc finger protein gene from sweetpotato, IbZFP1, is involved in salt and drought tolerance in transgenic Arabidopsis.Planta. 2016 Mar;243(3):783-97. doi: 10.1007/s00425-015-2443-9. Epub 2015 Dec 21. Planta. 2016. PMID: 26691387
-
Sweet Potato as a Key Crop for Food Security under the Conditions of Global Climate Change: A Review.Plants (Basel). 2023 Jun 30;12(13):2516. doi: 10.3390/plants12132516. Plants (Basel). 2023. PMID: 37447081 Free PMC article. Review.
Cited by
-
A comprehensive overview of omics-based approaches to enhance biotic and abiotic stress tolerance in sweet potato.Hortic Res. 2024 Jan 12;11(3):uhae014. doi: 10.1093/hr/uhae014. eCollection 2024 Mar. Hortic Res. 2024. PMID: 38464477 Free PMC article.
-
Molecular and biochemical responses of sesame (Sesame indicum L.) to rhizobacteria inoculation under water deficit.Front Plant Sci. 2024 Jan 18;14:1324643. doi: 10.3389/fpls.2023.1324643. eCollection 2023. Front Plant Sci. 2024. PMID: 38304453 Free PMC article.
-
Maize leaf yellowing gene ZmCAAX modulates growth and drought resistance by regulating abscisic acid contents through interaction with the ABA biosynthetic enzyme ZmNCED3.Plant Biotechnol J. 2025 Aug;23(8):3431-3450. doi: 10.1111/pbi.70147. Epub 2025 Jun 3. Plant Biotechnol J. 2025. PMID: 40458027 Free PMC article.
References
-
- Ceccarelli S., Grando S., Maatougui M., Michael M., Slash M., Haghparast R., et al. . (2010). Plant breeding and climate changes. J. Agric. Sci. 148, 627–637. doi: 10.1017/S0021859610000651 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

