Transplanted human induced pluripotent stem cells- derived retinal ganglion cells embed within mouse retinas and are electrophysiologically functional
- PMID: 36388952
- PMCID: PMC9646916
- DOI: 10.1016/j.isci.2022.105308
Transplanted human induced pluripotent stem cells- derived retinal ganglion cells embed within mouse retinas and are electrophysiologically functional
Abstract
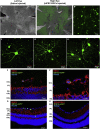
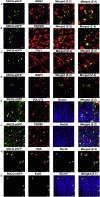
Glaucoma is an optic neuropathy characterized by permanent visual field loss caused by the death of retinal ganglion cells (RGCs) and it is the leading cause of irreversible blindness worldwide. Consequently, there is an unmet need for the development of new strategies for its treatment. We investigated RGC replacement therapy as a treatment for ganglion cell loss. Human-induced pluripotent stem cells (hiPSCs) were differentiated into mature, functional RGCs in vitro, labeled with AAV2.7m8-SNCG-eGFP, and transplanted intravitreally in wild-type 4-month-old C57BL/6J mice. Survival of the transplanted hiPSC-RGCs was assessed by color fundus photography and histological studies confirmed the localization of the transplanted hiPSC-RGCs within the retina. Two-photon live imaging of retinal explants and electrophysiological studies confirmed that the morphology and function of the transplanted hiPSC-RGCs were similar to native RGCs. These experiments will provide key strategies to enhance the efficiency of stem cell replacement therapy for neurodegenerative diseases, including glaucoma.
Keywords: Bioengineering; Biological sciences; Biotechnology; Cell biology; Stem cells research; Tissue engineering.
© 2022.
Conflict of interest statement
All affiliations are listed on the title page of the article. All funding sources for this study are listed in the “acknowledgments” section of the article. We, the authors and our immediate family members have no financial interests to declare. We, the authors and our immediate family members, have no positions to declare and are not members of the journal’s advisory board. We, the authors, have a patent related to this work, which is noted in the “declaration of interests” section of the article and on this form below, and we have noted the patents of immediate family members. “There are restrictions to the availability of the hiPSC-RGCs owing to patent pending on the differentiation protocol.”
Figures


References
-
- Aoki H., Hara A., Niwa M., Motohashi T., Suzuki T., Kunisada T. An in vitro mouse model for retinal ganglion cell replacement therapy using eye-like structures differentiated from ES cells. Exp. Eye Res. 2007;84:868–875. - PubMed
-
- Banin E., Obolensky A., Idelson M., Hemo I., Reinhardtz E., Pikarsky E., Ben-Hur T., Reubinoff B. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells (Dayton, Ohio) 2006;24:246–257. - PubMed
-
- Bellocchio E.E., Reimer R.J., Fremeau R.T., Jr., Edwards R.H. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science (New York, N.Y.) 2000;289:957–960. - PubMed
-
- Berg S., Kutra D., Kroeger T., Straehle C.N., Kausler B.X., Haubold C., Schiegg M., Ales J., Beier T., Rudy M., Eren K., Cervantes J.I., Xu B., Beuttenmueller F., Wolny A., Zhang C., Koethe U., Hamprecht F.A., Kreshuk A. Ilastik: interactive machine learning for (Bio)image analysis. Nat. Methods. 2019;16:1226–1232. - PubMed
-
- Chaffiol A., Caplette R., Jaillard C., Brazhnikova E., Desrosiers M., Dubus E., Duhamel L., Macé E., Marre O., Benoit P., Hantraye P., Bemelmans A.P., Bamberg E., Duebel J., Sahel J.A., Picaud S., Dalkara D. A new promoter allows optogenetic vision restoration with enhanced sensitivity in macaque retina. Mol. Ther. 2017;25:2546–2560. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

