Reduced immunogenicity of β-lactoglobulin by single amino acid substitution
- PMID: 36389282
- PMCID: PMC9652177
- DOI: 10.1007/s10616-022-00549-9
Reduced immunogenicity of β-lactoglobulin by single amino acid substitution
Abstract
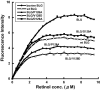
To reduce the immunogenicity of β-lactoglobulin (BLG), we prepared single amino acid substituted recombinant BLG mutants (BLG/P126A, BLG/V128D and BLG/D129A) in the methylotrophic yeast Pichia Pastris by fusion of the cDNA to the sequence coding for the α-factor signal peptide from Saccharomyces cerevisiae. Isoelectric points of single amino acid substituted BLGs were lower than that of native BLG. CD spectra indicated that the secondary structure of BLG had maintained native structure in single amino acid substituted BLGs. Fluorescence studies indicated that the conformation around Trp had not changed in single amino acid substituted BLGs. Anti-BLG antibody response was evaluated after immunization to C57BL/6 mice. Antibody response was reduced after immunization with BLG/P126A, BLG/V128D and BLG/D129A. And novel immunogenicity was not observed in the experiments. T cell proliferative response was evaluated in C57BL/6 mice, and it was clarified that BLG mutants also showed low response. Methods employed in this study was considered to be very effective to reduce immunogenicity of BLG.
Keywords: Protein engineering; Reduced immunogenicity; Single amino acid substitution; β-Lactoglobulin.
© The Author(s), under exclusive licence to Springer Nature B.V. 2022, Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors have no conflicts of interest to declare.
Figures







References
-
- Chen FM, Lee JH, Yang YH, Lin YT, Wang LC, Yu HH, Chiang BL. Analysis of α-lactalbumin-, β-lactoglobulin-, and casein-specific IgE among children with atopic diseases in a tertiary medical center in northern Taiwan. J Microbiol Immunol Infect. 2014;47:130–136. doi: 10.1016/j.jmii.2012.08.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources

