Next generations of CAR-T cells - new therapeutic opportunities in hematology?
- PMID: 36389658
- PMCID: PMC9650233
- DOI: 10.3389/fimmu.2022.1034707
Next generations of CAR-T cells - new therapeutic opportunities in hematology?
Abstract
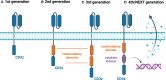
In recent years, the introduction of chimeric antigen receptor (CAR) T-cell therapies into clinics has been a breakthrough in treating relapsed or refractory malignancies in hematology and oncology. To date, Food and Drug Administration (FDA) has approved six CAR-T therapies for specific non-Hodgkin lymphomas, B-cell acute lymphoblastic leukemia, and multiple myeloma. All registered treatments and most clinical trials are based on so-called 2nd generation CARs, which consist of an extracellular antigen-binding region, one costimulatory domain, and a CD3z signaling domain. Unfortunately, despite remarkable overall treatment outcomes, a relatively high percentage of patients do not benefit from CAR-T therapy (overall response rate varies between 50 and 100%, with following relapse rates as high as 66% due to limited durability of the response). Moreover, it is associated with adverse effects such as cytokine release syndrome and neurotoxicity. Advances in immunology and molecular engineering have facilitated the construction of the next generation of CAR-T cells equipped with various molecular mechanisms. These include additional costimulatory domains (3rd generation), safety switches, immune-checkpoint modulation, cytokine expression, or knockout of therapy-interfering molecules, to name just a few. Implementation of next-generation CAR T-cells may allow overcoming current limitations of CAR-T therapies, decreasing unwanted side effects, and targeting other hematological malignancies. Accordingly, some clinical trials are currently evaluating the safety and efficacy of novel CAR-T therapies. This review describes the CAR-T cell constructs concerning the clinical application, summarizes completed and ongoing clinical trials of next-generation CAR-T therapies, and presents future perspectives.
Keywords: CAR-T cells; CRISPR; CRS; acute lymphoblastic leukemia; allogeneic; cytokine release syndrome; immunotherapy; lymphocyte.
Copyright © 2022 Tomasik, Jasiński and Basak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

