EBV-associated diseases: Current therapeutics and emerging technologies
- PMID: 36389670
- PMCID: PMC9647127
- DOI: 10.3389/fimmu.2022.1059133
EBV-associated diseases: Current therapeutics and emerging technologies
Abstract
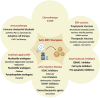
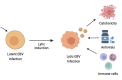
EBV is a prevalent virus, infecting >90% of the world's population. This is an oncogenic virus that causes ~200,000 cancer-related deaths annually. It is, in addition, a significant contributor to the burden of autoimmune diseases. Thus, EBV represents a significant public health burden. Upon infection, EBV remains dormant in host cells for long periods of time. However, the presence or episodic reactivation of the virus increases the risk of transforming healthy cells to malignant cells that routinely escape host immune surveillance or of producing pathogenic autoantibodies. Cancers caused by EBV display distinct molecular behaviors compared to those of the same tissue type that are not caused by EBV, presenting opportunities for targeted treatments. Despite some encouraging results from exploration of vaccines, antiviral agents and immune- and cell-based treatments, the efficacy and safety of most therapeutics remain unclear. Here, we provide an up-to-date review focusing on underlying immune and environmental mechanisms, current therapeutics and vaccines, animal models and emerging technologies to study EBV-associated diseases that may help provide insights for the development of novel effective treatments.
Keywords: EBV animal models; EBV therapeutics; EBV vaccines; EBV-associated diseases and cancers; high-throughput sequencing technologies; molecular mechanisms of EBV-host interactions.
Copyright © 2022 Chakravorty, Afzali and Kazemian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
New strategies and patent therapeutics in EBV-associated diseases.Mini Rev Med Chem. 2010 Sep;10(10):914-27. doi: 10.2174/138955710792007150. Mini Rev Med Chem. 2010. PMID: 21034415 Review.
-
Prophylactic and therapeutic strategies for Epstein-Barr virus-associated diseases: emerging strategies for clinical development.Expert Rev Vaccines. 2019 May;18(5):457-474. doi: 10.1080/14760584.2019.1605906. Epub 2019 Apr 24. Expert Rev Vaccines. 2019. PMID: 30987475 Review.
-
Association between Antibody Responses to Epstein-Barr Virus Glycoproteins, Neutralization of Infectivity, and the Risk of Nasopharyngeal Carcinoma.mSphere. 2020 Dec 2;5(6):e00901-20. doi: 10.1128/mSphere.00901-20. mSphere. 2020. PMID: 33268566 Free PMC article.
-
Vesicular Stomatitis Virus-Based Epstein-Barr Virus Vaccines Elicit Strong Protective Immune Responses.J Virol. 2022 May 11;96(9):e0033622. doi: 10.1128/jvi.00336-22. Epub 2022 Apr 11. J Virol. 2022. PMID: 35404082 Free PMC article.
-
Epstein-Barr virus-encoded microRNAs as regulators in host immune responses.Int J Biol Sci. 2018 Apr 5;14(5):565-576. doi: 10.7150/ijbs.24562. eCollection 2018. Int J Biol Sci. 2018. PMID: 29805308 Free PMC article. Review.
Cited by
-
Aquaporin-3 is down-regulated by LMP1 in nasopharyngeal carcinoma cells to regulate cell migration and affect EBV latent infection.Virus Genes. 2024 Oct;60(5):488-500. doi: 10.1007/s11262-024-02096-1. Epub 2024 Aug 5. Virus Genes. 2024. PMID: 39103702
-
Epstein-Barr Virus-Associated Aggressive Natural Killer Cell Leukemia: Challenges and Emerging Therapies.Cureus. 2024 Aug 6;16(8):e66338. doi: 10.7759/cureus.66338. eCollection 2024 Aug. Cureus. 2024. PMID: 39246900 Free PMC article.
-
Nationwide Longitudinal Annual Survey of HIV/AIDS Referral Hospitals in Japan From 1999 to 2021: Trend in Non-AIDS-defining Cancers Among Individuals Infected With HIV-1.J Acquir Immune Defic Syndr. 2024 May 1;96(1):1-10. doi: 10.1097/QAI.0000000000003389. Epub 2024 Apr 10. J Acquir Immune Defic Syndr. 2024. PMID: 38427920 Free PMC article.
-
The growing interests in Epstein-Barr virus: A bibliometric analysis of research trends, collaborations, and emerging hotspots.Infect Med (Beijing). 2025 Jun 22;4(3):100194. doi: 10.1016/j.imj.2025.100194. eCollection 2025 Sep. Infect Med (Beijing). 2025. PMID: 40703323 Free PMC article.
-
The Potential Role of Virus Infection in the Progression of Thyroid Cancer.World J Oncol. 2024 Jun;15(3):382-393. doi: 10.14740/wjon1830. Epub 2024 Apr 15. World J Oncol. 2024. PMID: 38751704 Free PMC article. Review.
References
-
- Longnecker R, Neipel F. Introduction to the human gamma-herpesviruses. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human herpesviruses: Biology, therapy, and immunoprophylaxis. Cambridge: Cambridge University Press; (2007). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

