Evaluation of humoral and cellular response to four vaccines against COVID-19 in different age groups: A longitudinal study
- PMID: 36389704
- PMCID: PMC9661524
- DOI: 10.3389/fimmu.2022.1021396
Evaluation of humoral and cellular response to four vaccines against COVID-19 in different age groups: A longitudinal study
Abstract
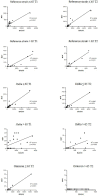
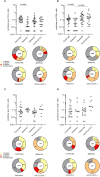
To date there has been limited head-to-head evaluation of immune responses to different types of COVID-19 vaccines. A real-world population-based longitudinal study was designed with the aim to define the magnitude and duration of immunity induced by each of four different COVID-19 vaccines available in Italy at the time of this study. Overall, 2497 individuals were enrolled at time of their first vaccination (T0). Vaccine-specific antibody responses induced over time by Comirnaty, Spikevax, Vaxzevria, Janssen Ad26.COV2.S and heterologous vaccination were compared up to six months after immunization. On a subset of Comirnaty vaccinees, serology data were correlated with the ability to neutralize a reference SARS-CoV-2 B strain, as well as Delta AY.4 and Omicron BA.1. The frequency of SARS-CoV-2-specific CD4+ T cells, CD8+ T cells, and memory B cells induced by the four different vaccines was assessed six months after the immunization. We found that mRNA vaccines are stronger inducer of anti-Spike IgG and B-memory cell responses. Humoral immune responses are lower in frail elderly subjects. Neutralization of the Delta AY.4 and Omicron BA.1 variants is severely impaired, especially in older individuals. Most vaccinees display a vaccine-specific T-cell memory six months after the vaccination. By describing the immunological response during the first phase of COVID-19 vaccination campaign in different cohorts and considering several aspects of the immunological response, this study allowed to collect key information that could facilitate the implementation of effective prevention and control measures against SARS-CoV-2.
Keywords: B-cell memory; COVID-19; cell-mediated immunity; serology; vaccines.
Copyright © 2022 Fedele, Trentini, Schiavoni, Abrignani, Antonelli, Baldo, Baldovin, Bandera, Bonura, Clerici, De Paschale, Fortunato, Gori, Grifantini, Icardi, Lazzarotto, Lodi, Mastroianni, Orsi, Prato, Restivo, Carsetti, Piano Mortari, Leone, Olivetta, Fiore, Di Martino, Brusaferro, Merler, Palamara and Stefanelli.
Conflict of interest statement
The authors declare that in this study the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet (2021) 397(10277):881–91. doi: 10.1016/S0140-6736(21)00432-3 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

