Humoral and cellular immunogenicity of COVID-19 booster dose vaccination in inflammatory arthritis patients
- PMID: 36389719
- PMCID: PMC9659732
- DOI: 10.3389/fimmu.2022.1033804
Humoral and cellular immunogenicity of COVID-19 booster dose vaccination in inflammatory arthritis patients
Abstract
Introduction: Previous studies have shown a reduction in the effectiveness of primary COVID-19 vaccination in patients with rheumatic diseases. However, limited data is available regarding the effectiveness of the COVID-19 vaccine booster dose, especially on cellular response. The study aimed to assess the humoral and cellular immunogenicity of a booster dose in patients with inflammatory arthritis (IA).
Patients and methods: 49 IA and 47 age and sex-matched healthy controls (HC) were included in a prospective cohort study. Both groups completed primary COVID-19 vaccination and after more than 180 days received a BNT162b2 booster shot. Humoral responses (level of IgG antibodies) and cellular responses (IFN-γ production) were assessed before and after 4 weeks from the booster dose of the vaccine.
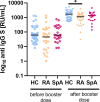
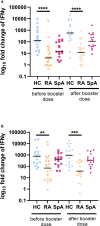
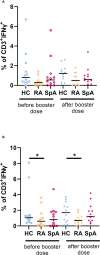
Results: After the booster dose, all participants showed an increased humoral response, although significantly reduced antibody levels were observed in IA patients compared to HC (p=0.004). The cellular response was significantly lower both before (p<0.001) and after (p<0.001) the booster dose in IA patients as compared to HC. Among the immunomodulatory drugs, only biological and targeted synthetic drugs lowered the humoral response after booster vaccination. However, the cellular response was decreased after all immunomodulatory drugs except IL-17 inhibitors and sulfasalazine.
Conclusion: Our data indicate that patients with rheumatic diseases present lower humoral and cellular responses after the COVID-19 booster vaccine in comparison to HC. This may translate into a recommendation for subsequent booster doses of the COVID-19 vaccine for rheumatic patients.
Keywords: COVID-19; arthritis; booster vaccine; cellular response; humoral response; immunogenicity.
Copyright © 2022 Wroński, Jaszczyk, Roszkowski, Felis-Giemza, Bonek, Kornatka, Plebańczyk, Burakowski, Lisowska, Kwiatkowska, Maśliński, Wisłowska, Massalska, Ciechomska and Kuca-Warnawin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ruddy JA, Connolly CM, Boyarsky BJ, Werbel WA, Christopher-Stine L, Garonzik-Wang J, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis (2021) 80:1351–2. doi: 10.1136/annrheumdis-2021-220656 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

