Interim efficacy and safety of PD-1 inhibitors in preventing recurrence of hepatocellular carcinoma after interventional therapy
- PMID: 36389724
- PMCID: PMC9650042
- DOI: 10.3389/fimmu.2022.1019772
Interim efficacy and safety of PD-1 inhibitors in preventing recurrence of hepatocellular carcinoma after interventional therapy
Abstract
Introduction: Locoregional interventional therapy including transcatheter arterial chemoembolization (TACE) and ablation are the current standard of treatment for early-to-mid-stage hepatocellular carcinoma (HCC). However, questions remain unanswered regarding the management of recurrence after locoregional treatment. PD-1 inhibitors can block inhibitory signals of T-cell activation and proliferation to reduce the recurrence. We conducted a single-arm phase 2 trial to evaluate the efficacy and safety of PD-1 inhibitors following locoregional interventional therapy in HCC patients with high recurrence risk guided by our novel scoring system.
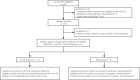
Methods: Patients enrolled initially treated by TACE combined with ablation, then willingly joined the experimental group. One month later, they received the anti-PD-1 adjuvant therapy (intravenous injection of 200 mg), which was repeated every 3 weeks for a total of 4 or 8 cycles. Within this same period, other patients were screened into the control group to match the experimental group by 1:1 based on the propensity score matching method (PSM). The primary endpoint was relapse-free survival (RFS). Secondary endpoints included overall survival (OS) recurrence modality, safety, and quality of life.
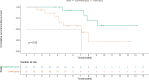
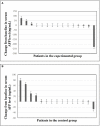
Result: At the time of data cutoff, the median RFS of the control group was 7.0 months while the experimental group had not reached it. Moreover, the 1-year RFS rate was 73.3% in the experimental group and 46.7% in the control group, showing a significant difference (P =0.02). The rate of local tumor progression in the experimental group was clearly lower than that in the control group (P = 0.027). Benefits associated with anti-PD-1 adjuvant therapy were observed in patients with multiple tumors and tumor size ≤2cm. Univariate and multivariate analyses demonstrated that anti-PD-1 adjuvant therapy was an independent favorable prognostic factor for RFS in HCC patients. The most frequent AE observed in this study was RCCEP, and other AEs included diarrhea, hepatotoxicity, rash, pruritus, and fatigue. The incidence of GRADE ≥3 AE and withdrawal in this study was low with no deaths recorded.
Conclusions: Interim analysis from the study suggest the addition of anti-PD-1 adjuvant therapy after TACE combined with ablation could significantly prolong RFS with controllable safety for early-to-mid-stage HCC patients with high recurrence risk.
Keywords: PD-1 inhibitors; TACE; ablation; hepatocellular carcinoma; immune; recurrence.
Copyright © 2022 Qiao, Wang, Hu, Zhang, Li, Sun, Yuan, Wang, Liu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. . Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatol (Baltimore Md) (2008) 47(1):82–9. doi: 10.1002/hep.21933 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

