A 9-mRNA signature measured from whole blood by a prototype PCR panel predicts 28-day mortality upon admission of critically ill COVID-19 patients
- PMID: 36389738
- PMCID: PMC9665875
- DOI: 10.3389/fimmu.2022.1022750
A 9-mRNA signature measured from whole blood by a prototype PCR panel predicts 28-day mortality upon admission of critically ill COVID-19 patients
Abstract
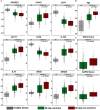
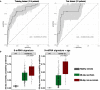
Immune responses affiliated with COVID-19 severity have been characterized and associated with deleterious outcomes. These approaches were mainly based on research tools not usable in routine clinical practice at the bedside. We observed that a multiplex transcriptomic panel prototype termed Immune Profiling Panel (IPP) could capture the dysregulation of immune responses of ICU COVID-19 patients at admission. Nine transcripts were associated with mortality in univariate analysis and this 9-mRNA signature remained significantly associated with mortality in a multivariate analysis that included age, SOFA and Charlson scores. Using a machine learning model with these 9 mRNA, we could predict the 28-day survival status with an Area Under the Receiver Operating Curve (AUROC) of 0.764. Interestingly, adding patients' age to the model resulted in increased performance to predict the 28-day mortality (AUROC reaching 0.839). This prototype IPP demonstrated that such a tool, upon clinical/analytical validation and clearance by regulatory agencies could be used in clinical routine settings to quickly identify patients with higher risk of death requiring thus early aggressive intensive care.
Keywords: 28-day mortality prediction; SARS-CoV-2 infection; immune response; personalized medicine; transcriptomic multiplex tool.
Copyright © 2022 Tardiveau, Monneret, Lukaszewicz, Cheynet, Cerrato, Imhoff, Peronnet, Bodinier, Kreitmann, Blein, Llitjos, Conti, Gossez, Buisson, Yonis, Cour, Argaud, Delignette, Wallet, Dailler, Monard, Brengel-Pesce, Venet and the RICO study group.
Conflict of interest statement
CT, VC, EC, KI, KB-P, EP, MBo, LK, SB, and J-FL are bioMérieux’s employees. EP, GM, and FV are co-inventors in patent applications covering the following markers: CX3CR1, CD127, IL10 and S100A9. bioFire – a bioMérieux company - holds patents on the technology. This does not alter the authors’ adherence to all the policies on sharing data and materials. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

