The carotid body: A novel key player in neuroimmune interactions
- PMID: 36389846
- PMCID: PMC9644854
- DOI: 10.3389/fimmu.2022.1033774
The carotid body: A novel key player in neuroimmune interactions
Abstract
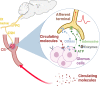
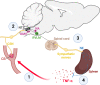
The idea that the nervous system communicates with the immune system to regulate physiological and pathological processes is not new. However, there is still much to learn about how these interactions occur under different conditions. The carotid body (CB) is a sensory organ located in the neck, classically known as the primary sensor of the oxygen (O2) levels in the organism of mammals. When the partial pressure of O2 in the arterial blood falls, the CB alerts the brain which coordinates cardiorespiratory responses to ensure adequate O2 supply to all tissues and organs in the body. A growing body of evidence, however, has demonstrated that the CB is much more than an O2 sensor. Actually, the CB is a multimodal sensor with the extraordinary ability to detect a wide diversity of circulating molecules in the arterial blood, including inflammatory mediators. In this review, we introduce the literature supporting the role of the CB as a critical component of neuroimmune interactions. Based on ours and other studies, we propose a novel neuroimmune pathway in which the CB acts as a sensor of circulating inflammatory mediators and, in conditions of systemic inflammation, recruits a sympathetic-mediated counteracting mechanism that appears to be a protective response.
Keywords: Carotid body; inflammation; neuroimmune interactions; neuroimmunomodulation; sympathetic nervous system.
Copyright © 2022 Katayama, Leirão, Kanashiro, Menani, Zoccal, Colombari and Colombari.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

