Next-Generation Sequencing and Bioinformatics-Based Protocol for the Full-Length CYP2E1 Gene Polymorphism Analysis
- PMID: 36393979
- PMCID: PMC9653044
- DOI: 10.2147/PGPM.S371709
Next-Generation Sequencing and Bioinformatics-Based Protocol for the Full-Length CYP2E1 Gene Polymorphism Analysis
Abstract
Introduction: Pharmacogenetics studies provide clinically relevant information on the identified associations between genetic variants and individual variability in drug response, which, in turn, offers great promise for guiding personalized drug therapy and clinical trial design. However, there is a lack of information concerning the evidence-based clinical annotations of specific CYP2E1 genetic variants.
Aim: To design and evaluate the next-generation sequencing-based method for full-length CYP2E1 gene polymorphism analysis.
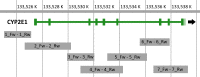
Materials and methods: Seven gene-specific oligonucleotide primer pairs targeting overlapping CYP2E1 gene fragments spanning all nine gene exons with interleaving introns, untranslated (UTR) and intergenic regions were designed. Human DNA samples (n = 3) were used as a training set to check the primer performance and to optimize the PCR conditions. The effectiveness of the developed target amplification and sequencing protocol was evaluated using the test set comprising human DNA samples (n = 3) obtained from tuberculosis patients. Sequencing data analysis was performed on the Galaxy online-based platform.
Results: The sequencing data quality was sufficient for the detection of genetic variants dispersed throughout the CYP2E1 gene with a high degree of confidence in fully covered regions achieving optimal reading depth of the targeted fragment with high base call accuracy.
Conclusion: Developed protocol can be applied in subpopulation-level association studies to determine whether single nucleotide variants (SNVs) or variant combinations from multiple regions of the CYP2E1 gene are of clinical significance.
Keywords: CYP2E1; cytochrome P450; next-generation sequencing; pharmacogenetics; single nucleotide variants.
© 2022 Igumnova et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures
References
-
- Stanford University and St. Jude Children’s Research Hospital. Clinical pharmacogenetics implementation consortium guidelines. Available from: https://cpicpgx.org/guidelines/. Accessed October 31, 2022.
LinkOut - more resources
Full Text Sources
Miscellaneous

