Improving the ex vivo expansion of human tumor-reactive CD8 + T cells by targeting toll-like receptors
- PMID: 36394017
- PMCID: PMC9659750
- DOI: 10.3389/fbioe.2022.1027619
Improving the ex vivo expansion of human tumor-reactive CD8 + T cells by targeting toll-like receptors
Abstract
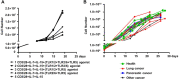
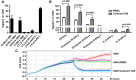
Toll-like receptors (TLRs) are important pattern recognition receptor(s) known to mediate the sensing of invading pathogens and subsequent immune responses. In this study, we investigate whether TLRs could be explored for the preparation of human CD8+ T cell products used in adoptive cell therapy (ACT). Following characterization of TLRs expression on human CD8+ T cells, we screened TLR-specific agonists for their ability to act in concert with anti-CD3 to stimulate the proliferation of these cells and corroborated the observed co-stimulatory effect by transcriptional profiling analyses. Consequently, we developed an optimal formulation for human CD8+ T cell amplification by combining CD3/CD28 antibody, interleukin 7 (IL-7), interleukin 15 (IL-15), and three agonists respectively targeting TLR1/2, TLR2/6, and TLR5. This new formulation performed better in amplifying PD-1+CD8+ T cells, a potential repertoire of tumor-reactive CD8+ T cells, from tumor patients than the conventional formulation. Importantly, the expanded CD8+ T cells showed restored functionality and consequently a robust anti-tumor activity in an in vitro co-culturing system. Together, our study established the utility of TLR agonists in ex vivo expansion of tumor-targeting CD8+ T cells, thus providing a new avenue toward a more effective ACT.
Keywords: adoptive cell therapy; agonists; cell proliferation; human PD1+ CD8+ T cells; toll-like receptors; tumor cell killing.
Copyright © 2022 Qiu, Wang, Zhu, Cheng, Xia, Jin, Qin, Zhang, Hu, Yan, Zhao, Zhang and Xu.
Conflict of interest statement
JX, XZ, and CQ are listed inventors of a Chinese patent ZL 1 0034922.8, and of a pending PCT application, numbered PCT/CN 2017/116342. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Expansion of CD3+CD8+PD1+ T lymphocytes and TCR repertoire diversity predict clinical responses to adoptive cell therapy in advanced gastric cancer.Am J Cancer Res. 2022 May 15;12(5):2203-2215. eCollection 2022. Am J Cancer Res. 2022. PMID: 35693097 Free PMC article.
-
Ex vivo expansion of dendritic-cell-activated antigen-specific CD4+ T cells with anti-CD3/CD28, interleukin-7, and interleukin-15: potential for adoptive T cell immunotherapy.Clin Immunol. 2006 Apr;119(1):21-31. doi: 10.1016/j.clim.2005.11.003. Epub 2006 Jan 9. Clin Immunol. 2006. PMID: 16406844
-
[Stimulatory and inhibitory signaling pathways of the T cell-APC interaction and the effect of TLR agonists on APCs].HNO. 2020 Dec;68(12):916-921. doi: 10.1007/s00106-020-00960-8. Epub 2020 Oct 30. HNO. 2020. PMID: 33128107 German.
-
Toll-like receptor-2/7-mediated T cell activation: An innate potential to augment CD8+ T cell cytokine production.Scand J Immunol. 2021 May;93(5):e13019. doi: 10.1111/sji.13019. Epub 2021 Jan 19. Scand J Immunol. 2021. PMID: 33377182 Review.
-
TLR10 and Its Role in Immunity.Handb Exp Pharmacol. 2022;276:161-174. doi: 10.1007/164_2021_541. Handb Exp Pharmacol. 2022. PMID: 34595581 Review.
Cited by
-
Anti-HIV Activity and Immunomodulatory Properties of Fractionated Crude Extracts of Alternaria alternata.Microorganisms. 2024 Jun 5;12(6):1150. doi: 10.3390/microorganisms12061150. Microorganisms. 2024. PMID: 38930532 Free PMC article.
-
Generation of TIL-based Cellular Products for Cancer Immunotherapy: Current Insights and the Challenges.Acta Naturae. 2025 Apr-Jun;17(2):15-27. doi: 10.32607/actanaturae.27559. Acta Naturae. 2025. PMID: 40771848 Free PMC article.
-
Safety and Clinical Response to Combined Immunotherapy with Autologous iNKT Cells and PD-1+CD8+ T Cells in Patients Failing First-line Chemotherapy in Stage IV Pancreatic Cancer.Cancer Res Commun. 2023 Jun 7;3(6):991-1003. doi: 10.1158/2767-9764.CRC-23-0137. eCollection 2023 Jun. Cancer Res Commun. 2023. PMID: 37377605 Free PMC article.
-
Glycyrrhiza polysaccharides may have an antitumor effect in γδT cells through gut microbiota and TLRs/NF-κB pathway in mice.FEBS Open Bio. 2024 Jun;14(6):1011-1027. doi: 10.1002/2211-5463.13800. Epub 2024 Apr 11. FEBS Open Bio. 2024. PMID: 38604998 Free PMC article.
References
-
- Cottalorda A., Mercier B. C., Mbitikon-Kobo F. M., Arpin C., Teoh D. Y., McMichael A., et al. (2009). TLR2 engagement on memory CD8(+) T cells improves their cytokine-mediated proliferation and IFN-gamma secretion in the absence of Ag. Eur. J. Immunol. 39 (10), 2673–2681. 10.1002/eji.200939627 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

