Immune checkpoint gene VSIR predicts patient prognosis in acute myeloid leukemia and myelodysplastic syndromes
- PMID: 36394080
- PMCID: PMC10028170
- DOI: 10.1002/cam4.5409
Immune checkpoint gene VSIR predicts patient prognosis in acute myeloid leukemia and myelodysplastic syndromes
Abstract
Background: Immune checkpoint proteins play critical functions during the immune response to cancer and have been targeted by immune checkpoint blockade therapy. V-domain Ig suppressor of T cell activation (VSIR) is one of these immune checkpoint genes and has been investigated extensively in recent years due to its conflicting roles in cancer immunity. Specifically, in acute myeloid leukemia (AML), the prognostic value of VSIR is debated.
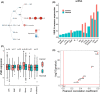
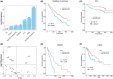
Results: In both patient tumor samples and cancer cell lines we find that VSIR has the highest expression in AML out of all cancer types and, in AML, has the highest expression out of all other immune checkpoint genes. Survival analysis indicated that AML patients with higher VSIR expression have significantly shorter survival than those patients with lower expression, even within established AML subgroups (e.g., FAB subtypes). Importantly, VSIR expression is predictive of progression from myelodysplastic syndromes (MDS) patients into AML, suggesting its potential role during the very early stage of AML development and progression. In addition to AML, VSIR also demonstrates prognostic values in other cancer types, including multiple myeloma and mesothelioma.
Conclusion: In summary, our analyses revealed the prognostic value of VSIR and its potential as a target for immunotherapy, especially in AML.
Keywords: VSIR; AML; MDS; prognosis.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures



Similar articles
-
Long non-coding RNA HOXB-AS3 promotes myeloid cell proliferation and its higher expression is an adverse prognostic marker in patients with acute myeloid leukemia and myelodysplastic syndrome.BMC Cancer. 2019 Jun 24;19(1):617. doi: 10.1186/s12885-019-5822-y. BMC Cancer. 2019. PMID: 31234830 Free PMC article.
-
Connect MDS/AML: design of the myelodysplastic syndromes and acute myeloid leukemia disease registry, a prospective observational cohort study.BMC Cancer. 2016 Aug 19;16:652. doi: 10.1186/s12885-016-2710-6. BMC Cancer. 2016. PMID: 27538433 Free PMC article.
-
Mutational profiling of therapy-related myelodysplastic syndromes and acute myeloid leukemia by next generation sequencing, a comparison with de novo diseases.Leuk Res. 2015 Mar;39(3):348-54. doi: 10.1016/j.leukres.2014.12.006. Epub 2014 Dec 20. Leuk Res. 2015. PMID: 25573287 Free PMC article.
-
Familial myelodysplastic syndrome/acute myeloid leukemia.Best Pract Res Clin Haematol. 2017 Dec;30(4):287-289. doi: 10.1016/j.beha.2017.10.002. Epub 2017 Oct 4. Best Pract Res Clin Haematol. 2017. PMID: 29156196 Free PMC article. Review.
-
Modulating the immune system as a therapeutic target for myelodysplastic syndromes and acute myeloid leukemia.Biochem Cell Biol. 2023 Dec 1;101(6):481-495. doi: 10.1139/bcb-2022-0374. Epub 2023 Aug 11. Biochem Cell Biol. 2023. PMID: 37566901 Review.
Cited by
-
Immune checkpoints represent a promising breakthrough in targeted therapy and prognosis of myelodysplastic syndrome.Heliyon. 2023 Sep 9;9(9):e19222. doi: 10.1016/j.heliyon.2023.e19222. eCollection 2023 Sep. Heliyon. 2023. PMID: 37810157 Free PMC article. Review.
-
Chemotherapy resistance in acute myeloid leukemia is associated with decreased anti-tumor immune response through MHC molecule and B7 family members.Discov Oncol. 2024 Jun 11;15(1):221. doi: 10.1007/s12672-024-01072-3. Discov Oncol. 2024. PMID: 38861194 Free PMC article.
-
Prognostic Analysis of Lactic Acid Metabolism Genes in Oral Squamous Cell Carcinoma.Int Dent J. 2024 Oct;74(5):1053-1063. doi: 10.1016/j.identj.2024.04.005. Epub 2024 Apr 26. Int Dent J. 2024. PMID: 38677972 Free PMC article.
-
Single-cell analysis reveals diversity of tumor-associated macrophages and their interactions with T lymphocytes in glioblastoma.Sci Rep. 2023 Nov 27;13(1):20874. doi: 10.1038/s41598-023-48116-2. Sci Rep. 2023. PMID: 38012322 Free PMC article.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7‐33. - PubMed
-
- DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology. Vol 2. Lippincott Williams & Wilkins; 2008.
-
- Varela B, Chuang C, Woll J, Bennett J. Modifications in the classification of primary myelodysplastic syndromes: the addition of a scoring system. Hematol Oncol. 1985;3:55‐63. - PubMed
-
- Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of hematological malignancies report of the clinical advisory committee meeting, Airlie house, Virginia, November 1997. Mod Pathol. 2000;13:193‐207. - PubMed
-
- Burnett A. The treatment of AML: current status and novel approaches. Hematology. 2005;10:50‐53. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

