Daily practices in chemotherapy for advanced gastric or gastroesophageal junction adenocarcinoma: METESTOMAC French prospective cohort
- PMID: 36394147
- PMCID: PMC10028027
- DOI: 10.1002/cam4.5354
Daily practices in chemotherapy for advanced gastric or gastroesophageal junction adenocarcinoma: METESTOMAC French prospective cohort
Abstract
Background: Around 50% of gastric cancers are diagnosed at an advanced stage. Several chemotherapy regimens are now internationally validated. Few data are available on the routine daily management of advanced gastric or gastroesophageal junction cancers. We aimed to describe chemotherapy practices, tolerance, and efficacy overall survival (OS) and Progression free survival (PFS) in a prospective French cohort.
Methods: Patients starting palliative chemotherapy were prospectively enrolled in 49 French centres. The primary objective was to report and describe patients' characteristics and treatment strategies. Secondary objectives were OS, PFS, objective response rate, adverse events rate, performance status deterioration during the chemotherapy.
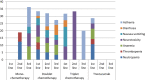
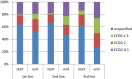
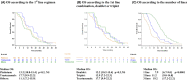
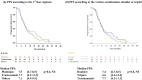
Results: A total of 182 patients were included; 179 were analysed. Most patients received platinium-based chemotherapy as the first treatment and FOLFIRI as second; 62.0% of patients received a second line, and 32.4% a third line. More than two thirds of Her2-positive patients were first treated with trastuzumab. The FOLFIRI regimen was the most frequently used second-line therapy. Median OS was 13.3 months, similar whatever the chemotherapy or combinations used in the first line. One- and 2-year OS increased with the number of chemotherapy lines received, from respectively 24.7% and 5.7% (1 line), to 46.9% and 12.4% (2 lines) and 88.1% and 29.9% (3 or more lines) (p < 0.0001).
Conclusion: Our study showed that treatment strategies in France are based on a succession of doublets, making it possible to offer a second and third line of treatment more often. This treatment strategy must be taken into account for future trials with immunotherapy combinations.
Keywords: cohort; gastric cancer; real life; strategy.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None for all the authors.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Defossez G, Le Guyader‐Peyrou S, Uhry Z, Grosclaude P, Colonna M, Dantony E, et al. Estimations nationales de l'incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018. Synthèse. Santé publique France, 2019. http://www.santepubliquefrance.fr/; https://www.e‐cancer.fr/.
-
- Verdecchia A, Guzzinati S, Francisci S, et al. Survival trends in European cancer patients diagnosed from 1988 to 1999. Eur J Cancer. 2009;45:1042‐1066. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous