Roles and Organization of BxpB (ExsFA) and ExsFB in the Exosporium Outer Basal Layer of Bacillus anthracis
- PMID: 36394311
- PMCID: PMC9765029
- DOI: 10.1128/jb.00290-22
Roles and Organization of BxpB (ExsFA) and ExsFB in the Exosporium Outer Basal Layer of Bacillus anthracis
Abstract
BxpB (also known as ExsFA) and ExsFB are an exosporium basal layer structural protein and a putative interspace protein of Bacillus anthracis that are known to be required for proper incorporation of the BclA collagen-like glycoprotein on the spore surface. Despite extensive similarity of the two proteins, their distribution in the spore is markedly different. We utilized a fluorescent fusion approach to examine features of the two genes that affect spore localization. The timing of expression of the bxpB and exsFB genes and their distinct N-terminal sequences were both found to be important for proper assembly into the exosporium basal layer. Results of this study provided evidence that the BclA nap glycoprotein is not covalently attached to BxpB protein despite the key role that the latter plays in BclA incorporation. Assembly of the BxpB- and ExsFB-containing outer basal layer appears not to be completely abolished in mutants lacking the ExsY and CotY basal layer structural proteins despite these spores lacking a visible exosporium. The BxpB and, to a lesser extent, the ExsFB proteins, were found to be capable of self-assembly in vitro into higher-molecular-weight forms that are stable to boiling in SDS under reducing conditions. IMPORTANCE The genus Bacillus consists of spore-forming bacteria. Some species of this genus, especially those that are pathogens of animals or insects, contain an outermost spore layer called the exosporium. The zoonotic pathogen B. anthracis is an example of this group. The exosporium likely contributes to virulence and environmental persistence of these pathogens. This work provides important new insights into the exosporium assembly process and the interplay between BclA and BxpB in this process.
Keywords: Bacillus anthracis; BclA; exosporium; immunofluorescence; promoters; protein assembly; spore.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
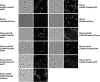







Similar articles
-
ExsY, CotY, and CotE Effects on Bacillus anthracis Outer Spore Layer Architecture.J Bacteriol. 2022 Nov 15;204(11):e0029122. doi: 10.1128/jb.00291-22. Epub 2022 Oct 4. J Bacteriol. 2022. PMID: 36194010 Free PMC article.
-
Localization and assembly of proteins comprising the outer structures of the Bacillus anthracis spore.Microbiology (Reading). 2009 Apr;155(Pt 4):1133-1145. doi: 10.1099/mic.0.023333-0. Microbiology (Reading). 2009. PMID: 19332815 Free PMC article.
-
The co-dependence of BxpB/ExsFA and BclA for proper incorporation into the exosporium of Bacillus anthracis.Mol Microbiol. 2011 Feb;79(3):799-813. doi: 10.1111/j.1365-2958.2010.07488.x. Mol Microbiol. 2011. PMID: 21255119 Free PMC article.
-
The Bacillus anthracis Exosporium: What's the Big "Hairy" Deal?Microbiol Spectr. 2015 Oct;3(5). doi: 10.1128/microbiolspec.TBS-0021-2015. Microbiol Spectr. 2015. PMID: 26542035 Review.
-
The Exosporium Layer of Bacterial Spores: a Connection to the Environment and the Infected Host.Microbiol Mol Biol Rev. 2015 Dec;79(4):437-57. doi: 10.1128/MMBR.00050-15. Microbiol Mol Biol Rev. 2015. PMID: 26512126 Free PMC article. Review.
Cited by
-
A new fluorescence-based approach for direct visualization of coat formation during sporulation in Bacillus cereus.Sci Rep. 2023 Sep 13;13(1):15136. doi: 10.1038/s41598-023-42143-9. Sci Rep. 2023. PMID: 37704668 Free PMC article.
-
Crystal structure and induced stability of trimeric BxpB: implications for the assembly of BxpB-BclA complexes in the exosporium of Bacillus anthracis.mBio. 2023 Aug 31;14(4):e0117223. doi: 10.1128/mbio.01172-23. Epub 2023 Jun 29. mBio. 2023. PMID: 37382447 Free PMC article.
References
-
- Gerhardt P. 1967. Cytology of Bacillus anthracis. Fed Proc 26:1504–1517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

