Expanded Proteomic Survey of the Human Parasite Leishmania major Focusing on Changes in Null Mutants of the Golgi GDP-Mannose/Fucose/Arabinopyranose Transporter LPG2 and of the Mitochondrial Fucosyltransferase FUT1
- PMID: 36394313
- PMCID: PMC9769760
- DOI: 10.1128/spectrum.03052-22
Expanded Proteomic Survey of the Human Parasite Leishmania major Focusing on Changes in Null Mutants of the Golgi GDP-Mannose/Fucose/Arabinopyranose Transporter LPG2 and of the Mitochondrial Fucosyltransferase FUT1
Abstract
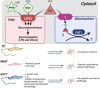
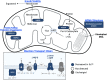
The trypanosomatid protozoan parasite Leishmania has a significant impact on human health globally. Understanding the pathways associated with virulence within this significant pathogen is critical for identifying novel vaccination and chemotherapy targets. Within this study we leverage an ultradeep proteomic approach to improve our understanding of two virulence-associated genes in Leishmania, encoding the Golgi mannose/arabinopyranose/fucose nucleotide-sugar transporter (LPG2) and the mitochondrial fucosyltransferase (FUT1). Using deep peptide fractionation followed by complementary fragmentation approaches with higher-energy collisional dissociation (HCD) and electron transfer dissociation (ETD) allowed the identification of over 6,500 proteins, nearly doubling the experimentally known Leishmania major proteome. This deep proteomic analysis revealed significant quantitative differences in both Δlpg2- and Δfut1s mutants with FUT1-dependent changes linked to marked alterations within mitochondrion-associated proteins, while LPG2-dependent changes impacted many pathways, including the secretory pathway. While the FUT1 enzyme has been shown to fucosylate peptides in vitro, no evidence for protein fucosylation was identified within our ultradeep analysis, nor did we observe fucosylated glycans within Leishmania glycopeptides isolated using hydrophilic interaction liquid chromatography (HILIC) enrichment. This work provides a critical resource for the community on the observable Leishmania proteome as well as highlighting phenotypic changes associated with LPG2 or FUT1, ablation of which may guide the development of future therapeutics. IMPORTANCE Leishmania is a widespread trypanosomatid protozoan parasite of humans, with ~12 million cases currently, ranging from mild to fatal, and hundreds of millions asymptomatically infected. This work advances knowledge of the experimental proteome by nearly 2-fold, to more than 6,500 proteins and thus provides a great resource to investigators seeking to decode how this parasite is transmitted and causes disease and to identify new targets for therapeutic intervention. The ultradeep proteomics approach identified potential proteins underlying the "persistence-without-pathology" phenotype of mutants with deletion of the Golgi nucleotide transporter LPG2, showing many alterations and several candidates. Studies of a rare mutant with deletion of the mitochondrial fucosyltransferase FUT1 revealed changes underlying its strong mitochondrial dysfunction but did not reveal examples of fucosylation of either peptides or N-glycans. This suggests that this vital protein's elusive target(s) may be more complex than the methods used could detect or that this target may not be a protein but perhaps another glycoconjugate or glycolipid.
Keywords: N-linked glycans; N-linked glycoconjugates; fucose; glycoproteome; kinetoplast; mitochondria; trypanosomatid protozoan parasite; ultradeep proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pigott DM, Bhatt S, Golding N, Duda KA, Battle KE, Brady OJ, Messina JP, Balard Y, Bastien P, Pratlong F, Brownstein JS, Freifeld CC, Mekaru SR, Gething PW, George DB, Myers MF, Reithinger R, Hay SI. 2014. Global distribution maps of the leishmaniases. Elife 3:e28051. doi:10.7554/eLife.02851. - DOI - PMC - PubMed
-
- Mannan SB, Elhadad H, Loc TTH, Sadik M, Mohamed MYF, Nam NH, Thuong ND, Hoang-Trong BL, Duc NTM, Hoang AN, Elhusseiny KM, Minh LHN, Quynh TTH, Nghia TLB, Mai Nhu Y, Tieu TM, Hirayama K, Huy NT, Hamano S. 2021. Prevalence and associated factors of asymptomatic leishmaniasis: a systematic review and meta-analysis. Parasitol Int 81:102229. doi:10.1016/j.parint.2020.102229. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

