peri-Acenoacene Ribbons with Zigzag BN-Doped Peripheries
- PMID: 36394460
- PMCID: PMC9716572
- DOI: 10.1021/jacs.2c06803
peri-Acenoacene Ribbons with Zigzag BN-Doped Peripheries
Abstract
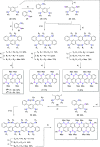
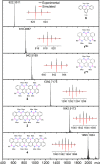
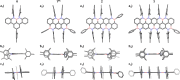
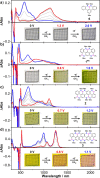
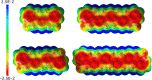
Here, we report the synthesis of BN-doped graphenoid nanoribbons, in which peripheral carbon atoms at the zigzag edges have been selectively replaced by boron and nitrogen atoms as BN and NBN motifs. This includes high-yielding ring closure key steps that, through N-directed borylation reaction using solely BBr3, allow the planarization of meta-oligoarylenyl precursors, through the formation of B-N and B-C bonds, to give ter-, quater-, quinque-, and sexi-arylenyl nanoribbons. X-ray single-crystal diffraction studies confirmed the formation of the BN and NBN motifs and the zigzag-edged topology of the regularly doped ribbons. Steady-state absorption and emission investigations at room temperature showed a systematic bathochromic shift of the UV-vis absorption and emission envelopes upon elongation of the oligoarylenyl backbone, with the nanoribbon emission featuring a TADF component. All derivatives displayed phosphorescence at 77 K. Electrochemical studies showed that the π-extension of the peri-acenoacene framework provokes a lowering of the first oxidative event (from 0.83 to 0.40 V), making these nanoribbons optimal candidates to engineer p-type organic semiconductors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


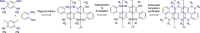




References
-
- Mishra S.; Yao X.; Chen Q.; Eimre K.; Gröning O.; Ortiz R.; Di Giovannantonio M.; Sancho-García J. C.; Fernández-Rossier J.; Pignedoli C. A.; Müllen K.; Ruffieux P.; Narita A.; Fasel R. Large Magnetic Exchange Coupling in Rhombus-Shaped Nanographenes with Zigzag Periphery. Nat. Chem. 2021, 13, 581–586. 10.1038/s41557-021-00678-2. - DOI - PubMed
-
- Ajayakumar M. R.; Fu Y.; Ma J.; Hennersdorf F.; Komber H.; Weigand J. J.; Alfonsov A.; Popov A. A.; Berger R.; Liu J.; Müllen K.; Feng X. Toward Full Zigzag-Edged Nanographenes: Peri-Tetracene and Its Corresponding Circumanthracene. J. Am. Chem. Soc. 2018, 140, 6240–6244. 10.1021/jacs.8b03711. - DOI - PubMed
-
- Ajayakumar M. R.; Ma J.; Feng X. Π-Extended Peri-Acenes: Recent Progress in Synthesis and Characterization. Eur. J. Org. Chem. 2022, 2022, e20210142810.1002/ejoc.202101428. - DOI
-
- Ajayakumar M. R.; Ma J.; Lucotti A.; Schellhammer K. S.; Serra G.; Dmitrieva E.; Rosenkranz M.; Komber H.; Liu J.; Ortmann F.; Tommasini M.; Feng X. Persistent Peri-Heptacene: Synthesis and In Situ Characterization. Angew. Chem., Int. Ed. 2021, 60, 13853–13858. 10.1002/anie.202102757. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

