Avelumab Plus Talazoparib in Patients With Advanced Solid Tumors: The JAVELIN PARP Medley Nonrandomized Controlled Trial
- PMID: 36394849
- PMCID: PMC9673022
- DOI: 10.1001/jamaoncol.2022.5228
Avelumab Plus Talazoparib in Patients With Advanced Solid Tumors: The JAVELIN PARP Medley Nonrandomized Controlled Trial
Abstract
Importance: Preclinical data suggest that poly(ADP-ribose) polymerase (PARP) inhibitors have synergistic activity when combined with immune checkpoint inhibitors (ICIs); however, it is unknown which tumor types or molecular subtypes may benefit from this combination.
Objective: To investigate responses associated with the combination of avelumab and talazoparib in different tumor types and/or molecular subtypes.
Design, setting, and participants: In this phase 1b and 2 basket nonrandomized controlled trial, patients with advanced solid tumors were enrolled in the following cohorts: non-small cell lung cancer (NSCLC); DNA damage response (DDR)-positive NSCLC; triple-negative breast cancer (TNBC); hormone receptor-positive, human epidermal growth factor receptor 2 (ERBB2)-negative, DDR-positive breast cancer; recurrent, platinum-sensitive ovarian cancer (OC); recurrent, platinum-sensitive, BRCA1/2-altered OC; urothelial cancer; metastatic castration-resistant prostate cancer (mCRPC); DDR-positive mCRPC; and BRCA1/2- or ATM-altered solid tumors. Data were analyzed between June 17, 2021, and August 6, 2021.
Interventions: All patients in phases 1b and 2 received avelumab plus talazoparib.
Main outcomes and measures: The phase 1b primary end point was dose-limiting toxic effects. The phase 2 primary end point was objective response, measured as objective response rate (ORR). Secondary end points included safety, time to response, duration of response (DOR), progression-free survival, time to prostate-specific antigen progression and PSA response of 50% or greater (for mCRPC), cancer antigen 125 response (for OC), pharmacokinetics, immunogenicity, and biomarkers.
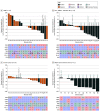
Results: A total of 223 patients (mean [SD] age, 63.2 [11.0] years; 117 [52.5%] men) were treated, including 12 patients in phase 1b and 211 patients in phase 2. The recommended phase 2 dose was avelumab 800 mg every 2 weeks plus talazoparib 1 mg once daily. In phase 2, the ORR was 18.2% (95% CI, 5.2%-40.3%) in patients with TNBC; 34.8% (95% CI, 16.4%-57.3%) in patients with HR-positive, ERBB2-negative, and DDR-positive BC; and 63.6% (95% CI, 30.8%-89.1%) in patients with platinum-sensitive, BRCA1/2-altered OC. Responses occurred more frequently in patients with BRCA1/2-altered tumors. Durable responses were observed in patients with TNBC (median [range] DOR, 11.1 [3.4-20.4] months); HR-positive, ERBB2-negative, and DDR-positive BC (median [range] DOR, 15.7 [3.9 to ≥20.6] months); and BRCA1/2-altered OC (median DOR not reached; range, 5.6 to ≥18.4 months). The most common grade 3 or greater treatment-related adverse events were anemia (75 patients [33.6%]), thrombocytopenia (48 patients [21.5%]), and neutropenia (31 patients [13.9%]).
Conclusions and relevance: This nonrandomized controlled trial found that ORRs for avelumab plus talazoparib were comparable with those with PARP inhibitor or ICI monotherapy. Prolonged DOR in patients with TNBC; HR-positive, ERBB2-negative, and DDR-positive BC; and BRCA1/2-altered OC warrant further investigation in randomized clinical trials. These data highlight the importance of prospective patient selection in future studies of ICI and PARP-inhibitor combinations.
Trial registration: ClinicalTrials.gov Identifier: NCT03330405.
Conflict of interest statement
Figures

Comment in
-
PARP inhibition and immunotherapy: a promising duo in fighting cancer.Transl Cancer Res. 2023 Sep 30;12(9):2433-2437. doi: 10.21037/tcr-23-726. Epub 2023 Aug 3. Transl Cancer Res. 2023. PMID: 37859734 Free PMC article. No abstract available.
Comment on
-
Combining PARP Inhibitor With Immunotherapy-Does the Promise of Preclinical Data Translate to Clinic?JAMA Oncol. 2023 Jan 1;9(1):25-27. doi: 10.1001/jamaoncol.2022.4591. JAMA Oncol. 2023. PMID: 36394835 No abstract available.
References
-
- Burtness B, Harrington KJ, Greil R, et al. ; KEYNOTE-048 Investigators . Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi:10.1016/S0140-6736(19)32591-7 - DOI - PubMed
-
- EMD Serono . Bavencio (avelumab) prescribing information. Accessed October 11, 2022. https://www.emdserono.com/us-en/pi/bavencio-pi.pdf
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

