Organocuprate Cross-Coupling Reactions with Alkyl Fluorides
- PMID: 36394939
- PMCID: PMC10502612
- DOI: 10.1021/acs.orglett.2c03775
Organocuprate Cross-Coupling Reactions with Alkyl Fluorides
Abstract
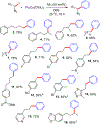
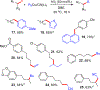
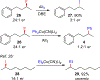
Cross-coupling of alkyl fluorides and organocuprates is accomplished via aluminum halide mediated C-F bond activation and subsequent Csp2-Csp3 and Csp3-Csp3 bond formation. Relatively mild conditions allow for smooth activation of notoriously challenging primary and secondary alkyl fluorides while competing alkyl chain rearrangement, HF elimination, and homocoupling reactions are effectively controlled. The utility and functional group tolerance are demonstrated with 23 examples and a variety of coupling products obtained in up to 88% yield.
Conflict of interest statement
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
Figures

References
-
- Terao J; Ikumi A; Kuniyasu H; Kambe N Ni- or Cu-Catalyzed Cross-Coupling Reaction of Alkyl Fluorides with Grignard Reagents. J. Am. Chem. Soc. 2003, 125, 5646–5647. - PubMed
-
- Matsubara K; Ishibashi T; Koga Y C-F Bond Cleavage Reactions of Fluoroalkanes with Magnesium Reagents and without Metal Catalysts. Org. Lett. 2009, 11, 1765–1768. - PubMed
-
- Blessley G; Holden P; Walker M; Brown JM; Gouverneur V Palladium-Catalyzed Substitution and Cross-Coupling of Benzylic Fluorides. Org. Lett. 2012, 14, 2754–2757. - PubMed
-
- Erickson LW; Lucas EL; Tollefson EJ; Jarvo ER Nickel-Catalyzed Cross-Electrophile Coupling of Alkyl Fluorides: Stereospecific Synthesis of Vinylcyclopropanes. J. Am. Chem. Soc. 2016, 138, 14006–15011. - PubMed
-
- Iwasaki T; Yamashita K; Kuniyasu H; Kambe N Co-Catalyzed Cross-Coupling Reaction of Alkyl Fluorides with Alkyl Grignard Reagents. Org. Lett. 2017, 19, 3691–3694. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

