Iron therapy mitigates chronic kidney disease progression by regulating intracellular iron status of kidney macrophages
- PMID: 36394951
- PMCID: PMC9870080
- DOI: 10.1172/jci.insight.159235
Iron therapy mitigates chronic kidney disease progression by regulating intracellular iron status of kidney macrophages
Abstract
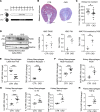
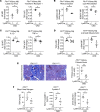
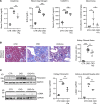
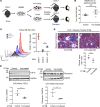
Systemic iron metabolism is disrupted in chronic kidney disease (CKD). However, little is known about local kidney iron homeostasis and its role in kidney fibrosis. Kidney-specific effects of iron therapy in CKD also remain elusive. Here, we elucidate the role of macrophage iron status in kidney fibrosis and demonstrate that it is a potential therapeutic target. In CKD, kidney macrophages exhibited depletion of labile iron pool (LIP) and induction of transferrin receptor 1, indicating intracellular iron deficiency. Low LIP in kidney macrophages was associated with their defective antioxidant response and proinflammatory polarization. Repletion of LIP in kidney macrophages through knockout of ferritin heavy chain (Fth1) reduced oxidative stress and mitigated fibrosis. Similar to Fth1 knockout, iron dextran therapy, through replenishing macrophage LIP, reduced oxidative stress, decreased the production of proinflammatory cytokines, and alleviated kidney fibrosis. Interestingly, iron markedly decreased TGF-β expression and suppressed TGF-β-driven fibrotic response of macrophages. Iron dextran therapy and FtH suppression had an additive protective effect against fibrosis. Adoptive transfer of iron-loaded macrophages alleviated kidney fibrosis, validating the protective effect of iron-replete macrophages in CKD. Thus, targeting intracellular iron deficiency of kidney macrophages in CKD can serve as a therapeutic opportunity to mitigate disease progression.
Keywords: Chronic kidney disease; Fibrosis; Macrophages; Nephrology.
Conflict of interest statement
Figures




Comment in
-
Filling the pool: possible renoprotective effects of repleting the kidney macrophage labile iron pool in CKD?Kidney Int. 2023 Jul;104(1):21-24. doi: 10.1016/j.kint.2023.03.022. Epub 2023 Apr 5. Kidney Int. 2023. PMID: 37068600 No abstract available.
References
-
- McMurray J, et al. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline... Accessed November 18, 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

