Reward insensitivity is associated with dopaminergic deficit in rapid eye movement sleep behaviour disorder
- PMID: 36395092
- PMCID: PMC10232265
- DOI: 10.1093/brain/awac430
Reward insensitivity is associated with dopaminergic deficit in rapid eye movement sleep behaviour disorder
Abstract
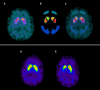
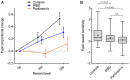
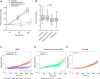
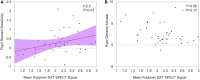
Idiopathic rapid eye movement sleep behaviour disorder (iRBD) has now been established as an important marker of the prodromal stage of Parkinson's disease and related synucleinopathies. However, although dopamine transporter single photon emission computed tomography (SPECT) has been used to demonstrate the presence of nigro-striatal deficit in iRBD, quantifiable correlates of this are currently lacking. Sensitivity to rewarding stimuli is reduced in some people with Parkinson's disease, potentially contributing to aspects of the neuropsychiatric phenotype in these individuals. Furthermore, a role for dopaminergic degeneration is suggested by the fact that reward insensitivity can be improved by dopaminergic medications. Patients with iRBD present a unique opportunity to study the relationship between reward sensitivity and early dopaminergic deficit in the unmedicated state. Here, we investigate whether a non-invasive, objective measure of reward sensitivity might be a marker of dopaminergic status in prodromal Parkinson's disease by comparing with SPECT/CT measurement of dopaminergic loss in the basal ganglia. Striatal dopaminergic deficits in iRBD are associated with progression to Parkinsonian disorders. Therefore, identification of a clinically measurable correlate of this degenerative process might provide a basis for the development of novel risk stratification tools. Using a recently developed incentivized eye-tracking task, we quantified reward sensitivity in a cohort of 41 patients with iRBD and compared this with data from 40 patients with Parkinson's disease and 41 healthy controls. Patients with iRBD also underwent neuroimaging with dopamine transporter SPECT/CT. Overall, reward sensitivity, indexed by pupillary response to monetary incentives, was reduced in iRBD cases compared with controls and was not significantly different to that in patients with Parkinson's disease. However, in iRBD patients with normal dopamine transporter SPECT/CT imaging, reward sensitivity was not significantly different from healthy controls. Across all iRBD cases, a positive association was observed between reward sensitivity and dopaminergic SPECT/CT signal in the putamen. These findings demonstrate a direct relationship between dopaminergic deficit and reward sensitivity in patients with iRBD and suggest that measurement of pupillary responses could be of value in models of risk stratification and disease progression in these individuals.
Keywords: Parkinson’s disease; REM sleep behaviour disorder; dopamine; reward.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

Comment in
-
Measurement of Pupillary Reward Sensitivity: A Tool for Risk Stratification in Isolated Rapid Eye Movement Behavioral Disorder?Mov Disord. 2023 Feb;38(2):199-200. doi: 10.1002/mds.29312. Epub 2023 Jan 9. Mov Disord. 2023. PMID: 36621936 No abstract available.
References
-
- Muhammed K, Husain M. Clinical significance of apathy in Parkinson’s disease. Cit EMJ Neurol. 2016;4:56–63.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

