Zanubrutinib Versus Ibrutinib in Relapsed/Refractory Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: Interim Analysis of a Randomized Phase III Trial
- PMID: 36395435
- PMCID: PMC9928683
- DOI: 10.1200/JCO.22.00510
Zanubrutinib Versus Ibrutinib in Relapsed/Refractory Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: Interim Analysis of a Randomized Phase III Trial
Abstract
Purpose: Zanubrutinib is a potent, irreversible next-generation Bruton tyrosine kinase (BTK) inhibitor designed to maximize BTK occupancy and minimize off-target kinase inhibition. We hypothesized that complete/sustained BTK occupancy may improve efficacy outcomes and increased BTK specificity may minimize off-target inhibition-related toxicities.
Patients and methods: ALPINE (ClinicalTrials.gov identifier: NCT03734016) is a global, randomized, open-label phase III study of zanubrutinib versus ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia. The primary end point was investigator-assessed overall response rate (ORR). The preplanned interim analysis was scheduled approximately 12 months after the first 415 patients were enrolled.
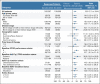
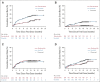
Results: Between November 1, 2018, and December 14, 2020, 652 patients were enrolled. We present the interim analysis of the first 415 enrolled patients randomly assigned to receive zanubrutinib (n = 207) or ibrutinib (n = 208). At 15 months of median follow-up, ORR (partial or complete response) was significantly higher with zanubrutinib (78.3%; 95% CI, 72.0 to 83.7) versus ibrutinib (62.5%; 95% CI, 55.5 to 69.1; two-sided P < .001). ORR was higher with zanubrutinib versus ibrutinib in subgroups with del(17p)/TP53 mutations (80.5% v 50.0%) and del(11q) (83.6% v 69.1%); 12-month progression-free survival in all patients was higher with zanubrutinib (94.9%) versus ibrutinib (84.0%; hazard ratio, 0.40; 95% CI, 0.23 to 0.69). Atrial fibrillation rate was significantly lower with zanubrutinib versus ibrutinib (2.5% v 10.1%; two-sided P = .001). Rates of cardiac events, major hemorrhages, and adverse events leading to treatment discontinuation/death were lower with zanubrutinib.
Conclusion: Zanubrutinib had a significantly higher ORR, lower atrial fibrillation rate, and improved progression-free survival and overall cardiac safety profile versus ibrutinib. These data support improved efficacy/safety outcomes with selective BTK inhibition.
Figures


References
-
- Eichhorst B, Robak T, Montserrat E, et al. : Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v78-v84, 2015. (suppl 5) - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. : Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 376:1164-1174, 2010 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

