Novel care pathway to optimise antimicrobial prescribing for uncomplicated community-acquired pneumonia: study protocol for a prospective before-after cohort study in the emergency department of a tertiary care Canadian children's hospital
- PMID: 36396301
- PMCID: PMC9677018
- DOI: 10.1136/bmjopen-2022-062360
Novel care pathway to optimise antimicrobial prescribing for uncomplicated community-acquired pneumonia: study protocol for a prospective before-after cohort study in the emergency department of a tertiary care Canadian children's hospital
Abstract
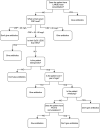
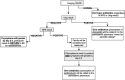
Introduction: Evidence-based recommendations for paediatric community-acquired pneumonia (CAP) diagnosis and management are needed. Uncomplicated CAP is often caused by respiratory viruses, especially in younger children; these episodes self-resolve without antibiotic treatment. Unfortunately, there are no clinical criteria that reliably discriminate between viral and bacterial disease, and so the majority of children diagnosed with CAP are given antibiotics-even though these will often not help and may cause harm. We have developed a novel care pathway that incorporates point-of-care biomarkers, radiographic patterns, microbiological testing and targeted follow-up. The primary study objective is to determine if the care pathway will be associated with less antimicrobial prescribing.
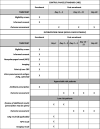
Methods and analysis: A prospective, before-after, study. Previously well children aged≥6 months presenting to a paediatric emergency department (ED) that have at least one respiratory symptom/sign, receive chest radiography, and are diagnosed with CAP by the ED physician will be eligible. Those with medical comorbidities, recently diagnosed pulmonary infection, or ongoing fever after≥4 days of antimicrobial therapy will be excluded. In the control (before) phase, eligible participants will be managed as per the standard of care. In the intervention (after) phase, eligible participants will be managed as per the novel care pathway. The primary outcome will be the proportion of participants in each phase who receive antimicrobial treatment for CAP. The secondary outcomes include: clinical cure; re-presentation to the ED; hospitalisation; time to resolution of symptoms; drug adverse events; caregiver satisfaction; child absenteeism from daycare/school; and caregiver absenteeism from work.
Ethics and dissemination: All study documentation has been approved by the Hamilton Integrated Research Ethics Board and informed consent will be obtained from all participants. Data from this study will be presented at major conferences and published in peer-reviewed publications to facilitate collaborations with networks of clinicians experienced in the dissemination of clinical guidelines.
Trial registration number: NCT05114161.
Keywords: diagnostic microbiology; paediatric A&E and ambulatory care; paediatric infectious disease & immunisation; respiratory infections.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JMP’s institution has received grant funding from MedImmune for an RSV research study. ML is on the WHO EML Working group, a Paladin Labs advisory board, and a Sunovion Pharmaceuticals advisory board.
Figures
Similar articles
-
Single-centre, open-label, randomised, trial to compare rapid molecular point-of-care streptococcal testing to standard laboratory-based testing for the management of streptococcal pharyngitis in children: study protocol.BMJ Open. 2021 Aug 11;11(8):e047271. doi: 10.1136/bmjopen-2020-047271. BMJ Open. 2021. PMID: 34380724 Free PMC article.
-
Predicting severe pneumonia in the emergency department: a global study of the Pediatric Emergency Research Networks (PERN)-study protocol.BMJ Open. 2020 Dec 2;10(12):e041093. doi: 10.1136/bmjopen-2020-041093. BMJ Open. 2020. PMID: 33268423 Free PMC article.
-
Short-Course Antimicrobial Therapy for Pediatric Community-Acquired Pneumonia: The SAFER Randomized Clinical Trial.JAMA Pediatr. 2021 May 1;175(5):475-482. doi: 10.1001/jamapediatrics.2020.6735. JAMA Pediatr. 2021. PMID: 33683325 Free PMC article. Clinical Trial.
-
Outpatient management of community-acquired pneumonia.Curr Opin Pulm Med. 2019 May;25(3):249-256. doi: 10.1097/MCP.0000000000000558. Curr Opin Pulm Med. 2019. PMID: 30585861 Review.
-
Childhood community-acquired pneumonia: A review of etiology- and antimicrobial treatment studies.Paediatr Respir Rev. 2018 Mar;26:41-48. doi: 10.1016/j.prrv.2017.06.013. Epub 2017 Jul 15. Paediatr Respir Rev. 2018. PMID: 28844414 Free PMC article. Review.
Cited by
-
Cost per episode of diarrhea and respiratory syncytial virus (RSV) in 128 low- and middle-income countries: how well do disease-specific and WHO-CHOICE estimates align?medRxiv [Preprint]. 2024 Jul 17:2024.07.17.24310217. doi: 10.1101/2024.07.17.24310217. medRxiv. 2024. PMID: 39072019 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
