Risk and response adapted therapy following autologous stem cell transplant in patients with newly diagnosed multiple myeloma (RADAR (UK-MRA Myeloma XV Trial): study protocol for a phase II/III randomised controlled trial
- PMID: 36396306
- PMCID: PMC9677008
- DOI: 10.1136/bmjopen-2022-063037
Risk and response adapted therapy following autologous stem cell transplant in patients with newly diagnosed multiple myeloma (RADAR (UK-MRA Myeloma XV Trial): study protocol for a phase II/III randomised controlled trial
Abstract
Introduction: Multiple myeloma is a plasma cell malignancy that accounts for 1%-2% of newly diagnosed cancers.At diagnosis, approximately 20% of patients can be identified, using cytogenetics, to have inferior survival (high-risk). Additionally, standard-risk patients, with detectable disease (minimal residual disease (MRD)-positive) postautologus stem cell transplant (ASCT), fare worse compared with those who do not (MRD-negative). Research is required to determine whether a risk-adapted approach post-ASCT could further improve patient outcomes.
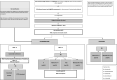
Methods: RADAR is a UK, multicentre, risk-adapted, response-guided, open-label, randomised controlled trial for transplant-eligible newly diagnosed multiple myeloma patients, using combinations of lenalidomide (R), cyclophosphamide (Cy), bortezomib (Bor), dexamethasone (D) and isatuximab (Isa).Participants receive RCyBorD(x4) induction therapy, followed by high-dose melphalan and ASCT. Post-ASCT, there are three pathways as follows:A phase III discontinuation design to assess de-escalating therapy in standard-risk MRD-negative patients. Participants receive 12 cycles of Isa maintenance. Those who remain MRD-negative are randomised to either continue or stop treatment.A phase II/III multiarm multistage design to test treatment strategies for treatment escalation in standard-risk MRD-positive patients. Participants are randomised to either; R, RBorD(x4) +R, RIsa, or RBorIsaD(x4) + RIsa.A phase II design to assess the activity of intensive treatment strategies in high-risk patients. Participants are randomised to RBorD(x4) +R or RBorIsaD(x4) + RIsa.1400 participants will be registered to allow for 500, 450 and 172 participants in each pathway. Randomisations are equal and treatment is given until disease progression or intolerance.
Ethics and dissemination: Ethical approval was granted by the London-Central Research Ethics Committee (20/LO/0238) and capacity and capability confirmed by the appropriate local research and development department for each participating centre prior to opening recruitment. Participant informed consent is required before trial registration and reconfirmed post-ASCT. Results will be disseminated by conference presentations and peer-reviewed publications.
Trial registration number: ISCRTN46841867.
Keywords: Cancer genetics; Clinical trials; Myeloma; Protocols & guidelines; STATISTICS & RESEARCH METHODS.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: ABC, KLR, DAC, AH, CO, LBai, LBar, AP and RL report grants and non-financial support from BMS/Celgene, grants and non-financial support from Merck Sharpe & Dohme, grants and non-financial support from Amgen, grants and non-financial support from Takeda, during the conduct of the trial. DAC also reports travel support from Celgene Corporation. KY, RDT, CB, GJ, GP, MC, BD, DM, GC, HA, KR,SA and AC have no conflicting interests to declare. MC declares, Bristol Myers Squibb- employee, Honoraria/travel support in the last 3 years from, Amgen, BMS/Celgene, Janssen, Takeda, Abbvie. CP declares BMS/Celgene Ad boards and speaker fees, Sanotif—Ad board, speaker fees, conference registration fees. JS reports Carrying out consultancy work (Advisory Board) for Sanofi. And an educational speaking engagement for Celgene/BMS RP declares; Honoraria—Jannsen, BMS, Abbvie, GSK. Consultancy: GSK, Janssen. Meeting support: Janssen, Takeda, BMS. RO declares- Janssen - advisory board, honoraria, Celegene— honoraria, Beigene - advisory board, honoraria, Astra Zeneca—honoraria. MK declares inter-relationships: AbbVie: consultancy; Amgen: honoraria; BMS/Celgene: consultancy, research funding (institution); GSK: consultancy; Janssen: consultancy, research funding (institution); Karyopharm: consultancy; Pfizer: consultancy; SeattleGenetics: consultancy; Takeda: consultancy; Sanofi: honoraria. MD reports owning stock in Abingdon Health. SQ is the founder and CSO of Achilles therapeutics a company developing T cell therapies for solid tumours.
Figures
References
-
- Cancer Research UK . Myeloma incidence statistics 2015, 2018. Available: http://www.cancerresearchuk.org/health-professional/cancer-statistics/st... [Accessed 21 Oct 2018].
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials